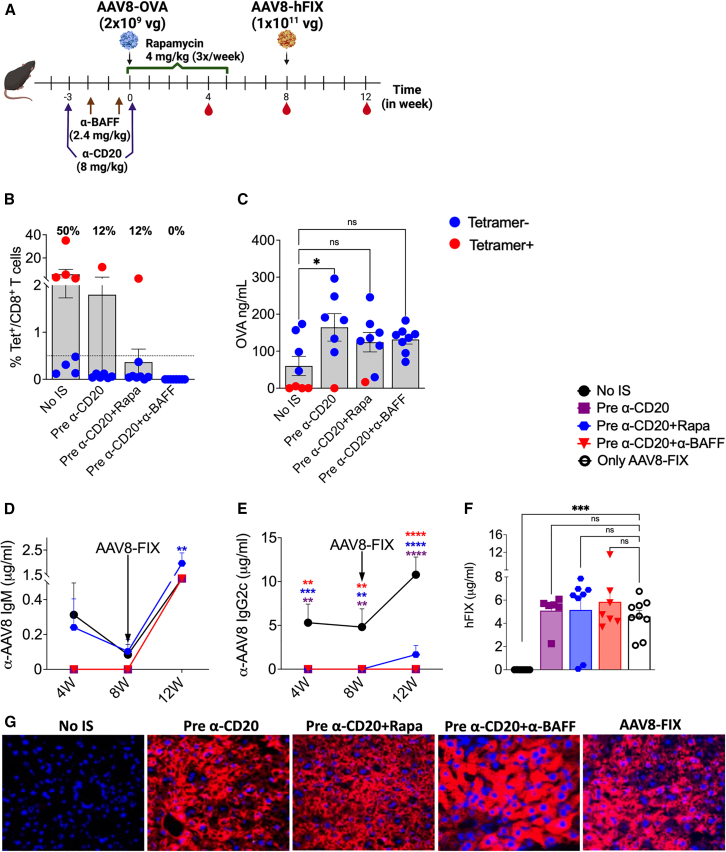
Figure 3.
IS before low-dose AAV administration abrogates NAb development
(A) Schematic representation of IS pretreatment regimens and AAV8 dosing. (B) CD8+ T cell response to the OVA transgene at 4 weeks post-AAV8-OVA administration (n = 7–8/group). (C) Circulating plasma levels of OVA at 4 weeks post-AAV8-OVA administration. (D) Longitudinal analysis of AAV8 capsid-specific IgM Ab levels in plasma samples post primary and -secondary AAV8 administrations. (E) AAV8 capsid-specific IgG2c Ab levels in plasma samples. (F) Circulating hFIX levels at 4 weeks post-re-administration. (G) Representative images of liver tissue for control (no-IS) and various IS treatment groups stained for hFIX (in red, 40× magnification) expression at 12 weeks post-re-administration. Statistical significance was calculated by one-way ANOVA (Dunnett’s multiple comparisons) for (B), (C), and (F), and two-way ANOVA for (D) and (E). ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001; ns, not significant.