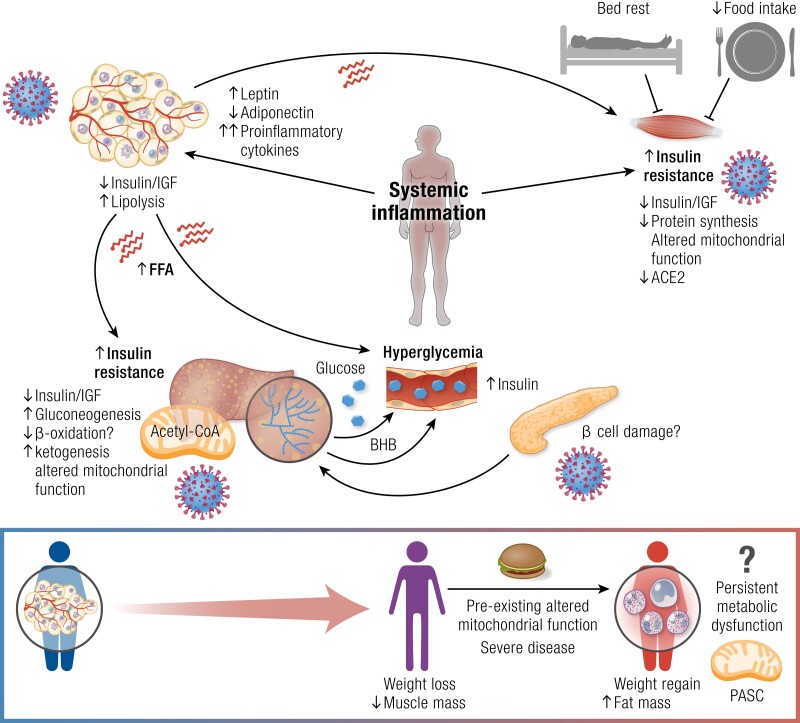
Figure 5.
Schematic representation of the effects of SARS-CoV-2 on organs involved in energy metabolism. SARS-CoV-2 infects the adipose tissue, enhancing preexisting inflammation in people with obesity/diabetes, which may result in an uncontrolled inflammatory response. COVID-19 and inflammation lead to adipocyte dysfunction, causing multiorgan insulin resistance, which in skeletal muscle is also promoted by elevations in interferon gamma that impair the insulin/IGF pathway, and downregulation of the angiotensin-converting enzyme 2 (ACE2) receptor. In adipose tissue, insulin resistance and possibly endothelial dysfunction result in increased lipolysis. Free fatty acids (FFA) that are released into the circulation may further worsen insulin resistance and, in the liver, may lead to lipid accumulation and promote hepatic gluconeogenesis, which is also enhanced by hyperinsulinemia secondary to insulin resistance and SARS-CoV-2-induced Golgi protein 73 (GP73) production. Altered mitochondrial function in the liver may impair fatty acid β oxidation and ketogenesis, which increases with increased COVID-19 severity, but is blunted in comparison to acute respiratory distress syndrome resulting from influenza virus. Muscle disuse and disease-related malnutrition result in muscle mass loss and further impairment of glucose disposal. Hyperglycemia in patients with COVID-19 may result from these mechanisms, and it is likely that only individuals who are predisposed to impaired glucose metabolism, possibly because of preexisting metabolic dysfunction, will develop long-term hyperglycemia and metabolic dysfunction, with COVID-19 acting as a second hit. In these persons, especially those who had severe COVID-19, weight regain with preferential fat catch-up during recovery may further worsen metabolic health, leading to persistent metabolic dysfunction and post-acute sequelae of COVID-19 (PASC). Abbreviations: ACE2, angiotensin-converting enzyme 2; BHB, beta-hydroxybutyrate; FFA, free fatty acids.