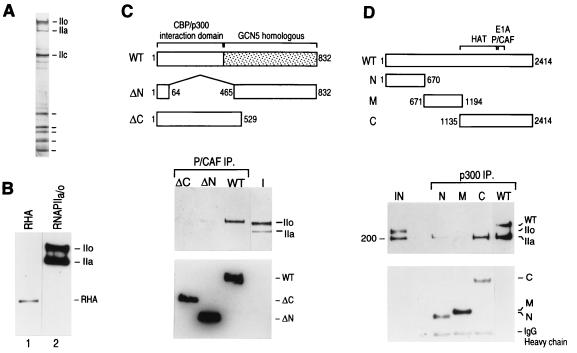
FIG. 5.
(A) Silver-stained gel of core RNAPII containing the phosphorylated (IIo) and nonphosphorylated (IIa) forms of the large subunit that was used in the interaction experiments shown in panels C and D. (B) Western blot analysis reveals the absence of RHA in the RNAPII preparation used in the interaction experiments. Lane 1 shows a Western blot of a crude fraction containing RHA, and lane 2 shows a Western blot of the RNAPII (50% of input) preparation used in the interaction studies. Blots were analyzed for RHA and subsequently reprobed for the largest subunit of RNAPII (IIa and IIo). (C) PCAF directly interacts with the phosphorylated form of RNAPII. Different deletion mutant polypeptides of PCAF (77) were immobilized on beads through their N-terminal FLAG tags and were incubated with a mixture of phosphorylated and nonphosphorylated forms of RNAPII (I; 10% of input). Immunoprecipitates (IP) were extensively washed as described in Materials and Methods and were analyzed by Western blotting using anti-CTD antibodies (top gel) and anti-FLAG antibodies (bottom gel). The diagram above the blots shows the different deletion PCAF proteins that were used in the assay. WT, wild type. (D) p300 directly interacts with the nonphosphorylated form of RNAPII through its C-terminal domain. Experiments were performed as described for panel C. The diagram at the top illustrates the different truncated p300 proteins (53) used. The top blot shows the results of Western blotting with anti-CTD and anti-FLAG antibodies; the bottom blot shows the results of Western blotting with anti-FLAG antibodies. IgG, immunoglobulin G.