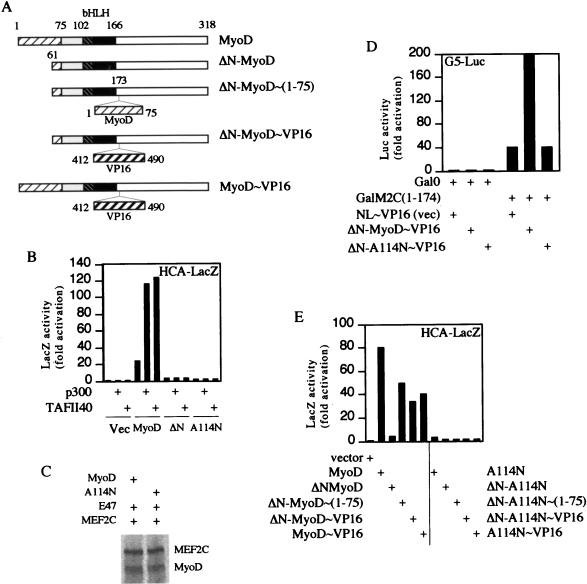
FIG. 4.
The A114N mutant fails to respond to coactivators of MyoD. (A) Schematic maps of the MyoD wild-type and mutated peptides used to acquire the data shown in panels B, D, and E. ▨, amino-terminal activation domain; ▧, DNA binding domain; ■, HLH dimerization domain. The grey box and white box do not symbolize any specific functional domains. (B) Residual transactivation activity of the A114N peptide cannot be potentiated by p300 or TAFII40; 1.5 μg of each plasmid was transfected. Vec, vector; ΔN, ΔN-MyoD∼(1-75). (C) The A114N mutant and MyoD interact with MEF2C equally well. Proteins synthesized by in vitro transcription-translation were mixed and immunoprecipitated with an antibody against the carboxyl terminus of MyoD. (D) Interaction of A114N with MEF2C [GalM2C(1–174)] is not reconstituted in a mammalian two-hybrid assay; 1 μg of each plasmid was used. (E) The A114N mutant fails to transactivate even when supplemented with VP16 activation domain. All transfections were done in NIH 3T3 cells and repeated at least three times; 2 μg of each plasmid was used. ΔN-MyoD and ΔN-A114N, MyoD and A114N proteins each lacking N-terminal aa 3 to 56; ΔN-MyoD∼VP16, ΔN∼MyoD with VP16 activation domain (aa 412 to 490) inserted between MyoD residues 173 and 174; ΔN-MyoD∼(1–75) and ΔN-A114N∼(1–75); ΔN-MyoD and ΔN-A114N with MyoD activation domain (aa 1 to 75) inserted between MyoD residues 173 and 174.