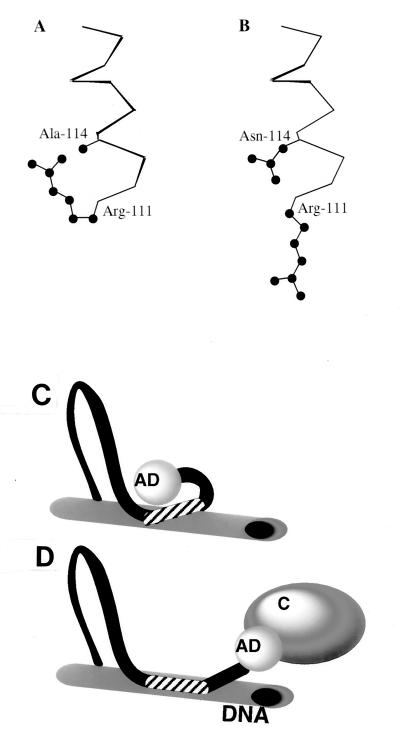
FIG. 5.
(A and B) Molecular modeling reveals different basic-domain conformations in wild-type MyoD (A) and the A114N mutant (B). α-Carbon traces of residues 111 and 114 are presented. Note the drastically different conformations of Arg-111. (C and D) Schematic representation of changes in MyoD structure following binding to DNA. Binding of MyoD basic domain (striped area) to a target DNA site (C) is associated with a distinctive change of conformational structure that is propagated to the activation domain (AD), allowing interaction with transcriptional coactivators (labeled as “C” in panel D). In panel C, the AD is sketched as interacting with the basic domain, an inference drawn from the inhibitory effects of basic domain elements shown in Table 2. DNA binding by the A114N mutant also keeps the AD in the conformation shown in panel C.