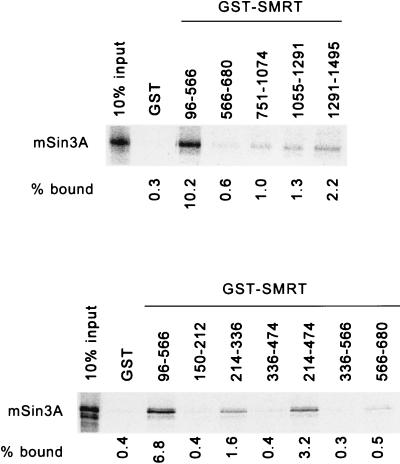
FIG. 2.
Localization of an interaction site for mSin3A within the SMRT polypeptide. Full-length, radiolabeled mSin3A was synthesized in vitro by a coupled transcription-translation protocol. The radiolabeled mSin3A protein was then incubated with various domains of SMRT, expressed in bacteria as GST fusion proteins, and immobilized on a glutathione-agarose column (the SMRT amino acids represented in each GST fusion are denoted above the panels; GST refers to a nonrecombinant GST construct employed as a negative control). The radiolabeled mSin3A protein bound by each GST-SMRT construct was eluted with soluble glutathione, resolved by SDS-PAGE, and visualized and quantified by PhosphorImager analysis. The percentage of the input mSin3A bound by each GST fusion is indicated below each lane.