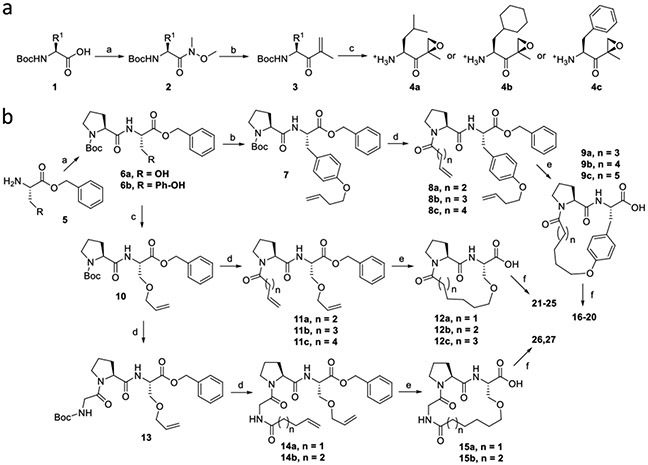
SCHEME 1. Synthetic strategy for macrocyclic analogs of DB-310.
Reagents and Conditions: a. (a) HN(CH3)OCH3, HOBt, EDCI.HCl, DIPEA, DCM, rt, 12 h; (b) Isopropenylmagnesium bromide, THF, −78 °C, 12 h; (c) i. Benzonitrile, H2O2, DIPEA, methanol, 0 °C to rt, 2 h. ii. TFA, DCM, rt, 1 h, then evaporated and vacuum-dried. b. (a) HBTU, HOBt, DIPEA, DCM, rt, 18 h; (b) Potassium carbonate, 4-Bromo-1-butane, DMF, rt, 6 h; (c) Allyl methyl carbonate, Pd(PPh3)4, THF, 60 °C, 3 h; (d) i. TFA, DCM, rt, 1 h, then evaporated and dried. ii. N-Boc glycine (for compound 13) or alkenyl carboxylic acid, HBTU, HOBt, DIPEA, DCM, rt, 18 h; (e) i. Grubb’s second-generation catalyst, toluene, 90 °C, 1 h, purified by flash column chromatography. ii. H2, Pd/C, methanol, rt, 1 h; (f) Amine deprotected epoxy ketone (4a, 4b or 4c), HBTU, HOBt, DIPEA, DCM, rt, 18 h.