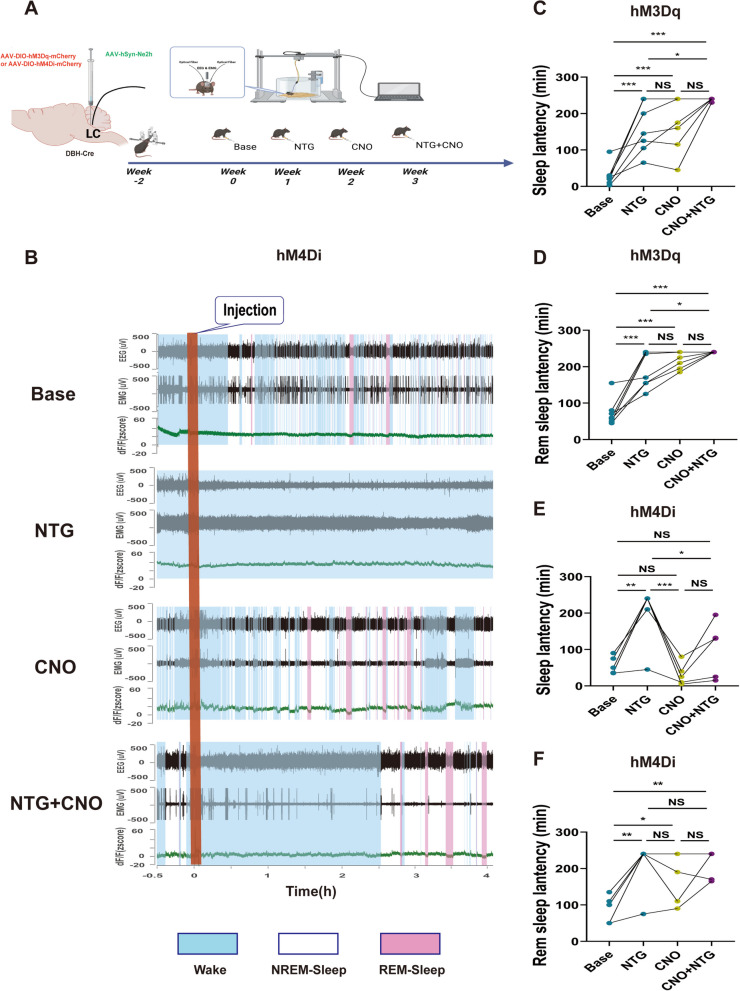
Fig. 6.
Chemogenetic modulation of noradrenergic neuron activity in the LC significantly influences the impact of acute pain on sleep architecture (n=6). A Schematic representation of the timeline depicting the acute administration of NTG and the utilization of chemogenetic techniques, leading to modifications in sleep architecture. B A representative image illustrating simultaneous recordings of EEG, EMG, and neuronal activity (Ne2h) in a DBH-Cre mouse highlights the significant influence of CNO on AAV-DIO-hM4Di-mCherry noradrenergic neurons, revealing its impact on the modulation of sleep architecture in response to acute pain. C and D Statistical trends of the effects of the activation of noradrenergic neurons on sleep architecture in response to acute pain. E and F Statistical trends of the effects of the inhibition of noradrenergic neurons on sleep architecture in response to acute pain. Data are expressed as the mean ± SEM. Two-way ANOVA with repeated measures for multiple comparisons. * P < 0.05; ** P < 0.01; ***P < 0.001. (C: Base vs. NTG P < 0.001, vs. CNO P < 0.001, vs. CNO+NTG P < 0.001; NTG vs. CNO P = 0.944, vs. CNO+NTG P = 0.022; CNO vs. CNO+NTG P = 0.069. D: Base vs. NTG P < 0.001, vs. CNO P < 0.001, vs. CNO+NTG P < 0.001; NTG vs. CNO P = 0.264, vs. CNO+NTG P = 0.025; CNO vs. CNO+NTG P = 0.587. E: Base vs. NTG P = 0.003, vs. CNO P = 0.839, vs. CNO+NTG P = 0.529; NTG vs. CNO P < 0.001, vs. CNO+NTG P = 0.037; CNO vs. CNO+NTG P = 0.173. F: Base vs. NTG P = 0.002, vs. CNO P = 0.021, vs. CNO+NTG P = 0.002; NTG vs. CNO P = 0.551, vs. CNO+NTG P = 0.998; CNO vs. CNO+NTG P = 0.459)