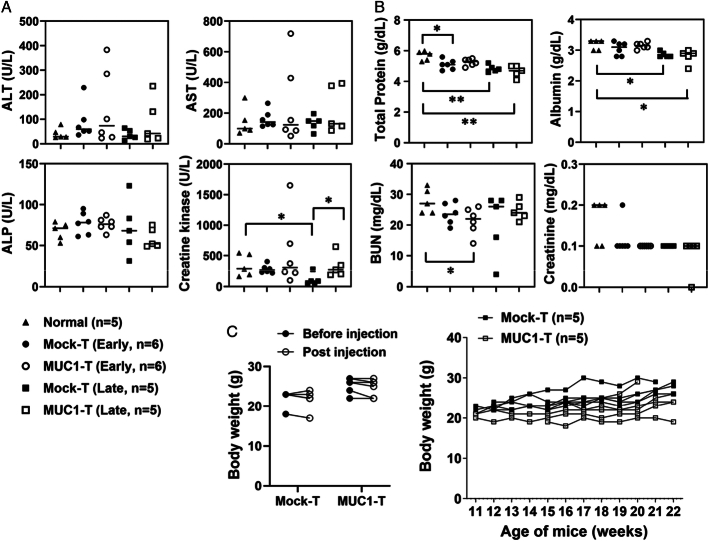
FIGURE 7.
MUC1 CAR T cells are well tolerated in spontaneous MMT mice. A and B, The mouse sera were collected to evaluate CAR T-cell toxicity for liver panel (A) and renal panel (B) as described in “Materials and Methods.” Normal, sera from normal MUC1.Tg mice; n = 5. Early, sera from spontaneous MMT mice 6 days after receiving one dose of T cells; n = 6 for each group. Late, sera from spontaneous MMT mice at the survival endpoint as in Figure 6B; n = 5 picked from each group. The statistical comparison was performed between normal and each T-cell treatment group, and between mock-T and MUC1-T at an early stage or at a late stage. The horizontal bar marks the median value in each group. The legends for (A) and (B) are shared and shown at the bottom (A). C, No significant body weight changes after T-cell treatment. Mice were the same as for (A and B) and no statistical difference was achieved. Left: mice were injected with mock T cells or MUC1 CAR T cells. Body weights were recorded right before T-cell injection (before injection) and on day 6 endpoint (after injection). Right: mice were from Figure 6B and body weights were recorded during the T-cell treatment course. *P <0.05, **P <0.01 (unpaired t tests). CAR indicates chimeric antigen receptor; MUC1, mucin 1. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen.