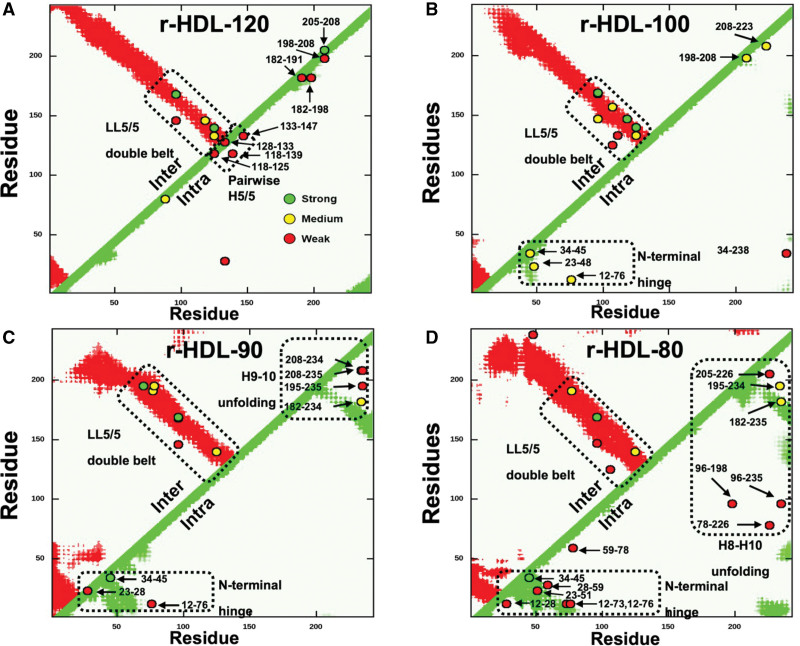
Figure 2.
Contact maps (A–D) of the intermolecular (Inter) and intramolecular (Intra) APOA1 (apolipoprotein A1) cross-links detected by tandem mass spectrometry (MS/MS) in different sizes of r-HDL. A, r-HDL-120. B, r-HDL-100. C, r-HDL-90. D, r-HDL-80. Red regions and green regions indicate the allowable distance of intermolecular and intramolecular peptide contacts (15.1 Å), respectively, in a molecular dynamics simulation of the LL5/5 double-belt model of APOA1.27 Cross-links (o) between APOA1 residues are labeled. Semiquantitative estimates of the strengths of interactions between residues were based on ion currents (Table S2), and they are indicated by the colors of the circles (green, strong; yellow, medium; and red, weak). Note that we detected multiple intramolecular cross-linked peptides in the helix 8 (H8) to helix 10 (H10) region and the helix 9 (H9) to helix 10 region of the C-terminus of APOA1 of r-HDL-80 and r-HDL-90 particles, respectively, that are inconsistent with the classic double-belt model. This indicates that the C-terminus of APOA1 has increased conformational freedom and does not assume the double-belt conformation in that region. In contrast, the intramolecular cross-linked peptides detected in that region of the 2 largest sizes of high-density lipoprotein are consistent with the double-belt model. r-HDL indicates reconstituted high-density lipoprotein.