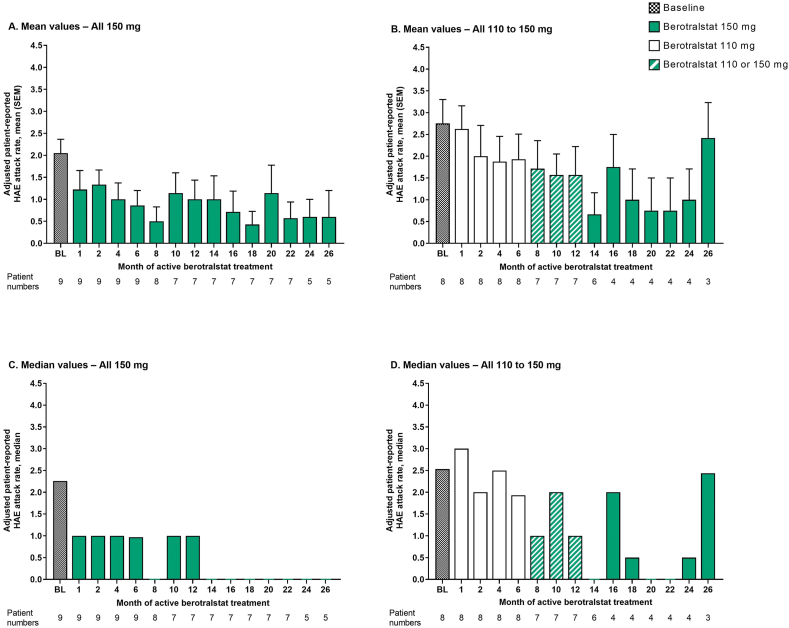
Fig. 2.
Adjusted patient-reported HAE attack rates from baseline to 26 months of active berotralstat treatment for patients who entered Part 2 of APeX-J (n = 17). A. Mean (SEM) values for the All 150 mg group, B. Mean (SEM) values for the All 110 to 150 mg group, C. Median values for the All 150 mg group, and D. Median values for the All 110 to 150 mg group. Baseline attack rates are based on the number of attacks that occurred during the 56-day run-in period before study entry. For patients who received berotralstat following placebo, months are adjusted according to the date of the first berotralstat dose. Two patients in the All 110 to 150 mg group who experienced attacks during the last month were not on study for the full 28 days of the month and, as such, their attack rate for this month was calculated using a denominator smaller than 28 days. BL, baseline; HAE, hereditary angioedema; SEM, standard error of the mean