Figure 2.
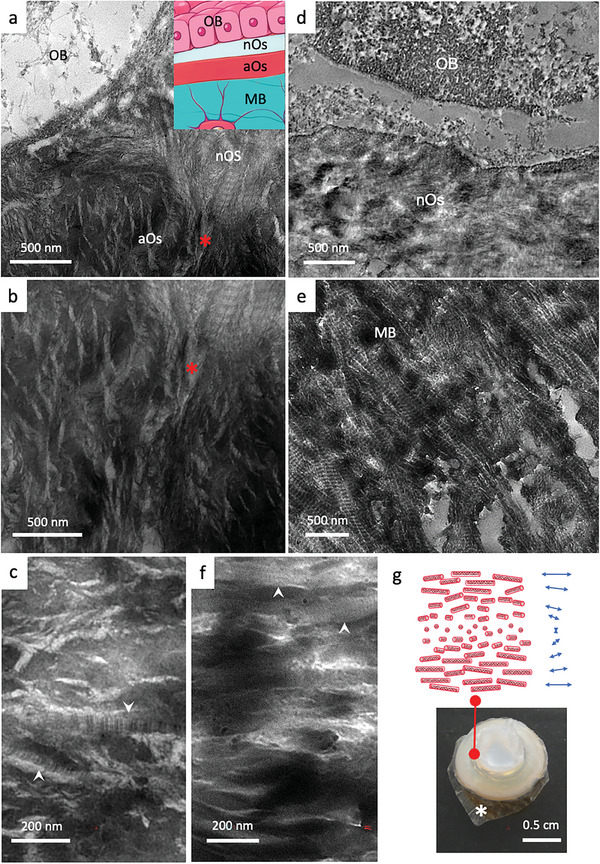
Investigations by TEM of collagen ultrastructure in bone tissues (osteoid, mature bone) and in synthetic acidic collagen mesophases which form physical gels. a,b) TEM micrographs of osteoid domains (nOs and aOs) in close proximity with an osteoblast (OB) (the red * identified the same feature in adjacent regions) show that collagen fibrils are typical for the nOs but hardly found in the aOs. The inset in a is a schematic representation of a histological bone section colored with Goldner's trichrome stain. From top to down, the monolayer that forms osteoblasts (OB), the neutral osteoid (nOs), the acidic osteoid tissue (aOs), and mature bone (MB). c) aOs appears denser to electrons with parallel packing of non‐fibrillar structures. Some fibrils are rarely observed (white arrows). Micrograph in d) shows the newly secreted nOs with randomly oriented cross‐striated fibrils in contact with an OB. In e) the classical anisotropic packing of mineralized cross‐striated fibrils in MB is observed, i.e., arced pattern revealed by an oblique section performed on the twisted plywood organization. f) in vitro lyotropic acidic solution of collagen molecules concentrated in the presence of apatite ion precursors exhibit textural similarities with aOs domains (c) are observed with locally aligned domains of collagen molecules and occasional fibrils (white arrows in c and f). g) A schematic representation of the spatial organization of collagen molecules shows a long‐range helical organization of collagen triple helices (cholesteric phase). Image of a highly concentrated acidic solution of collagen molecules (≈250 mg mL−1) containing apatite ion precursors (calcium, phosphate, and carbonate ions). Collagen solutions are no longer fluid at such concentration owing to their high viscosity. The sample is supported by a dialysis membrane (*).
