Fig. 1 |. Elimination process and interaction of renal-clearable engineered nanoparticles in the kidneys.
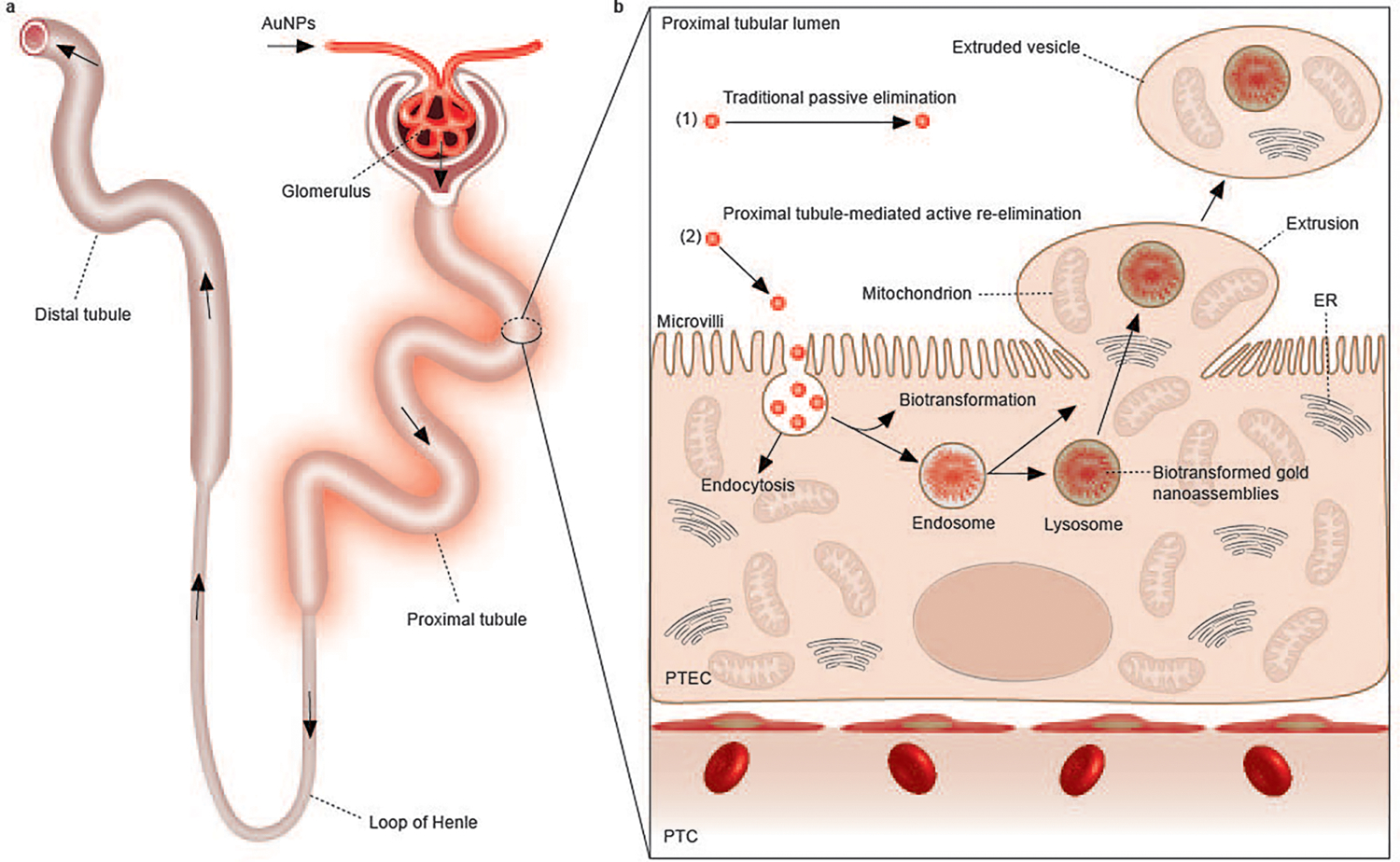
a, Renal-clearable engineered nanoparticles (for example, AuNPs) will be first filtered through the glomerulus. The filtered nanoparticles will then enter the PTs, followed by travel through the loop of Henle, the distal tubules and the collecting ducts, and eventually entering the bladder through the ureters. Among all the components of the nephron, the PT is the most active site of uptake of the filtered nanoparticles due to the densely packed microvilli on its luminal surface. b, In addition to the traditional passive elimination of nanoparticles through the kidneys, with little involvement of cellular internalization and metabolism (1), we discovered a PT-mediated active re-elimination pathway of AuNPs involving endocytosis, biotransformation in the endosomes/lysosomes and cellular elimination through organelle extrusion on the luminal membrane of PTs (2). The 2–3 nm AuNPs can be internalized by PTECs through endocytosis. The endocytosed AuNPs are then partially biotransformed into 200–300 nm, large nanoassemblies inside endosomes/lysosomes. By squeezing ~5 μm balloon-like extrusions through dense microvilli, transporting intact gold-containing endosomes or lysosomes into the extrusions along with mitochondria or other organelles and pinching off the extrusions from the cell membrane into the lumen, PTECs re-eliminated endocytosed AuNPs, including those large biotransformed nanoassemblies, into the urine, and they were further cleared out of the body afterward. PTC, peritubular capillary.
