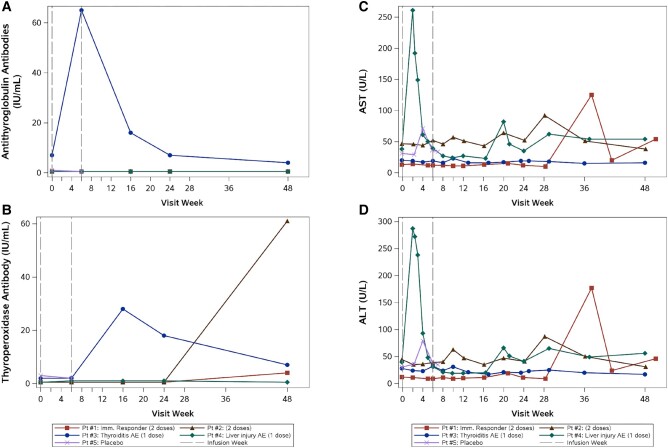
Figure 1.
Antithyroglobulin antibodies (A) and thyroperoxidase antibodies (B) over time for each participant. Participant (Pt) #1 and Pt #2 received 2 doses of cemiplimab. Pt #3 and #4 received a single dose of cemiplimab due to suspected adverse events. Pt #5 received 1 placebo dose. Pt #3 (shown in blue, thyroiditis immune-related adverse event [AE]) had a detectable pretreatment antithyroglobulin antibody level and thyroperoxidase antibody level with an increase in both measures after cemiplimab infusion. Alanine aminotransferase (ALT; C) and aspartate aminotransferase (AST; D) over time for each participant. Pt #4 (shown in green, liver injury AE) experienced increased AST and ALT at week 2. Elevated AST and ALT (grade 1–2) recurred for this participant at weeks 20, 29, and 48, attributed to ongoing alcohol use.