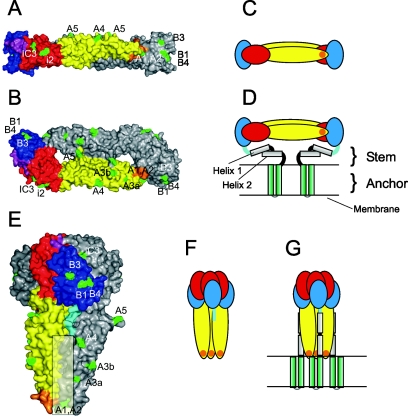
FIG. 1.
Native dimeric and trimeric postfusion structures of the TBEV E protein. (A, B) Surface representations of the X-ray crystal structure of the TBEV sE dimer (18) in a side view (A) and a top view (B). The three domains are color coded in one of the monomeric subunits: red, domain I; yellow, domain II; blue, domain III. The linker between domain I and III is shown in purple, the fusion peptide loop in orange. Positions of amino acid mutations that lead to the loss of MAb binding to virions are indicated in green and designated according to the corresponding MAb. (C, D) Schematic drawings of the E dimer in its truncated form (C) and with the stem-anchor region attached (D). (E, F, G) Representations of the trimeric low-pH form of E (4) analogous to those shown for the native forms in panels A to D. The linker between domain III and helix 1 of the stem is shown in light blue, and the postulated site of interaction of domains II with the stem is indicated by a shaded rectangle in panel E.