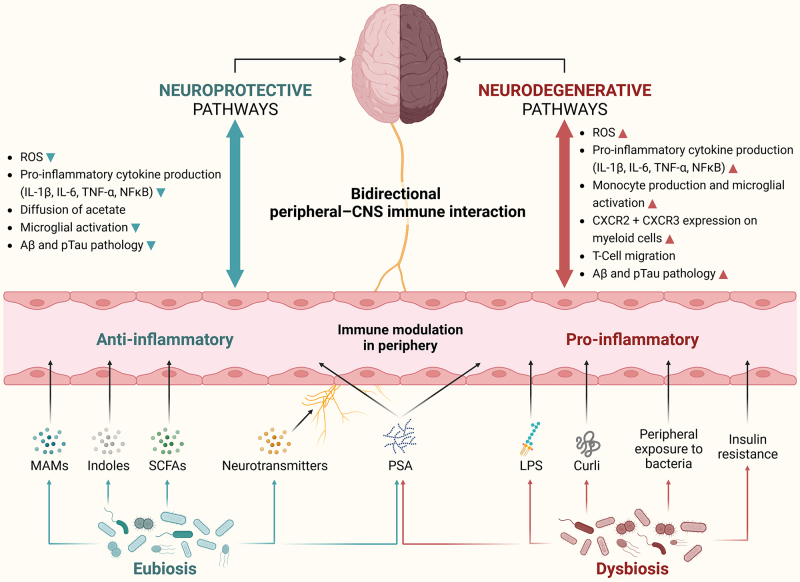
Figure 4.
Neuroprotective and neurodegenerative pathways of microbiota-gut-brain axis describing complex bidirectional crosstalk between gut microbes, gut-derived metabolites, and the local (brain) and peripheral (blood) immune system. Gut dysbiosis initiates a proinflammatory environment in the periphery by instigating an immune response against microbial surface molecules (LPS, Curli, and PSA). This disruption adversely impacts the local blood-brain-barrier ecosystem, leading to an excessive influx of proinflammatory T-cell types, activation of M1 microglia, and an increased expression of chemokine receptors on myeloid cells. These events ultimately culminate in the accumulation of ROS and the development of Aβ and pTau pathologies associated with neurodegeneration. In contrast, a state of gut eubiosis establishes an anti-inflammatory environment in the periphery through the production of beneficial gut-derived metabolites (indoles, SCFAs, and neurotransmitters), as well as beneficial bacterial molecules (MAMs and other PSA). This fortifies the blood-brain-barrier ecosystem by facilitating the diffusion of specific metabolites such as acetate and preventing the overactivation of microglia. Ultimately, these conditions confer neuroprotective effects by reducing ROS, Aβ, and pTau pathologies. Aβ: Amyloid beta; CNS: central nervous system; CXCR: C-X-C chemokine receptors; IL: interleukins; LPS: lipopolysaccharide; MAMs: microbial anti-inflammatory molecules; NFκB: nuclear factor kappa B; PSA: polysaccharide A; pTau: phosphorylated tau protein; ROS: reactive oxygen species; SCFAs: short-chain fatty acids; TNF-α: tumor necrosis factor alpha.