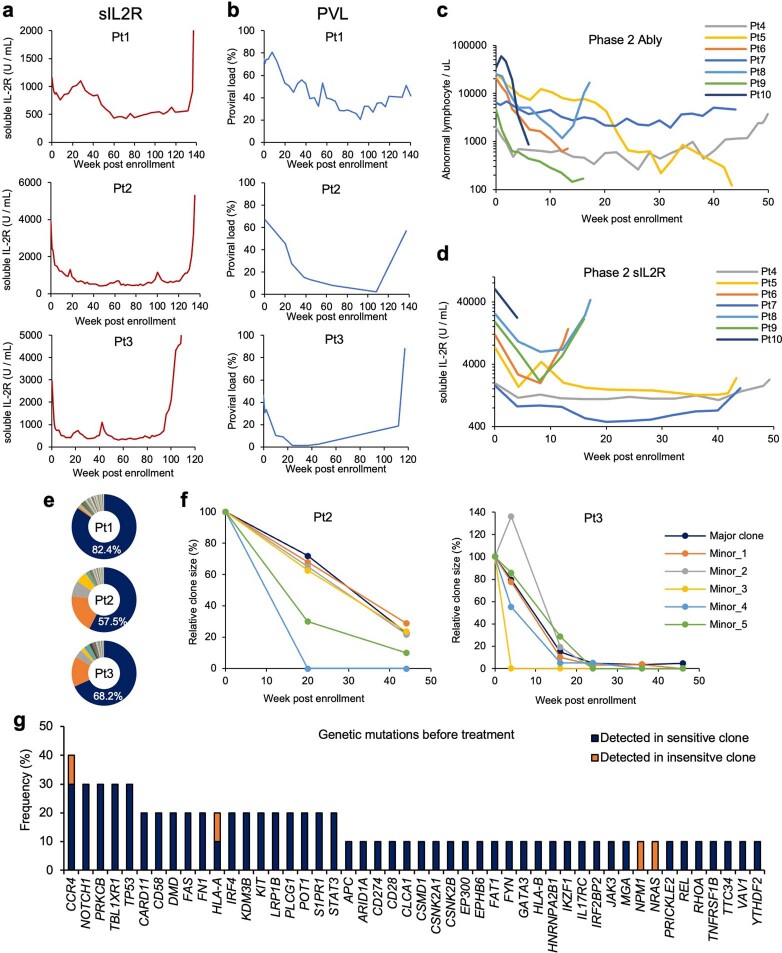
Extended Data Fig. 1. Clinical efficacy and genomic profiling in valemetostat trials.
a, b, Changes over time in soluble IL2 receptor (sIL-2R) (a) and proviral loads (PVL) (b) of three cases in valemetostat phase 1 study. c, d, Changes in abnormal lymphocytes (c) and sIL-2R (d) of 7 cases in valemetostat phase 2 study. e, Pie charts show baseline clonalities in three patients. The clonality of HTLV-1-infected cells before valemetostat treatment was calculated as the population size of each clone by counting the extracted reads at host-provirus junction sites using high-throughput sequencing based mapping of proviral integration sites. f, Changes over time in the size of top 5 clones in Pt2 (left) and Pt3 (right) after treatment with valemetostat. g, Frequency of somatic mutations detected by targeted genome sequencing in valemetostat phase 1 and 2 studies (n = 10, biologically independent samples).