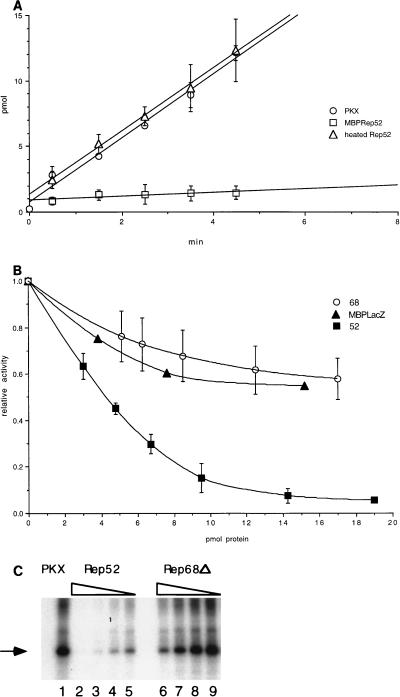
FIG. 7.
Inhibition of PrKX trans and autophosphorylation activity. (A) Incubation of purified cos His-PrKX with Kemptide and γ-32P-labeled ATP showed transfer of 32P to the Kemptide substrate. Addition of a 7-fold molar excess of MBP-Rep52 inhibited greater than 90% of this kinase activity. Rep52 heated to 65°C for 10 min prior to addition did not inhibit kinase activity. (B) The kinetics of inhibition of PrKX by MBP-Rep68Δ and MBP-LacZ were compared with that for MBP-Rep52. Increasing amounts of each protein were added to the kinase reaction mixture containing 2.6 pmol of His-PrKX protein each. The resulting kinase activity was then compared with that of a control reaction mixture containing only PrKX, and percent inhibition was determined. Similar levels of PrKX activity were measured in the presence of either MBP-Rep68Δ or MBP-LacZ. (C) Inhibition of autophosphorylation of PrKX, which was measured by incubation of 100 nM PrKX in the presence of [γ-32P]ATP, results in phosphorylation of the 42-kDa His-PrKX fusion protein, as indicated by the arrow (lane 1). This activity is inhibited upon addition of fourfold molar excess of MBP-Rep52 (lane 2). Twofold serial dilution of MBP-Rep52 (200, 100, and 50 nM) eventually restored some activity (lanes 3, 4, and 5). A similar titration of MBP-Rep68Δ showed only slight inhibition at the highest concentrations tested (lanes 6 to 9).