Abstract
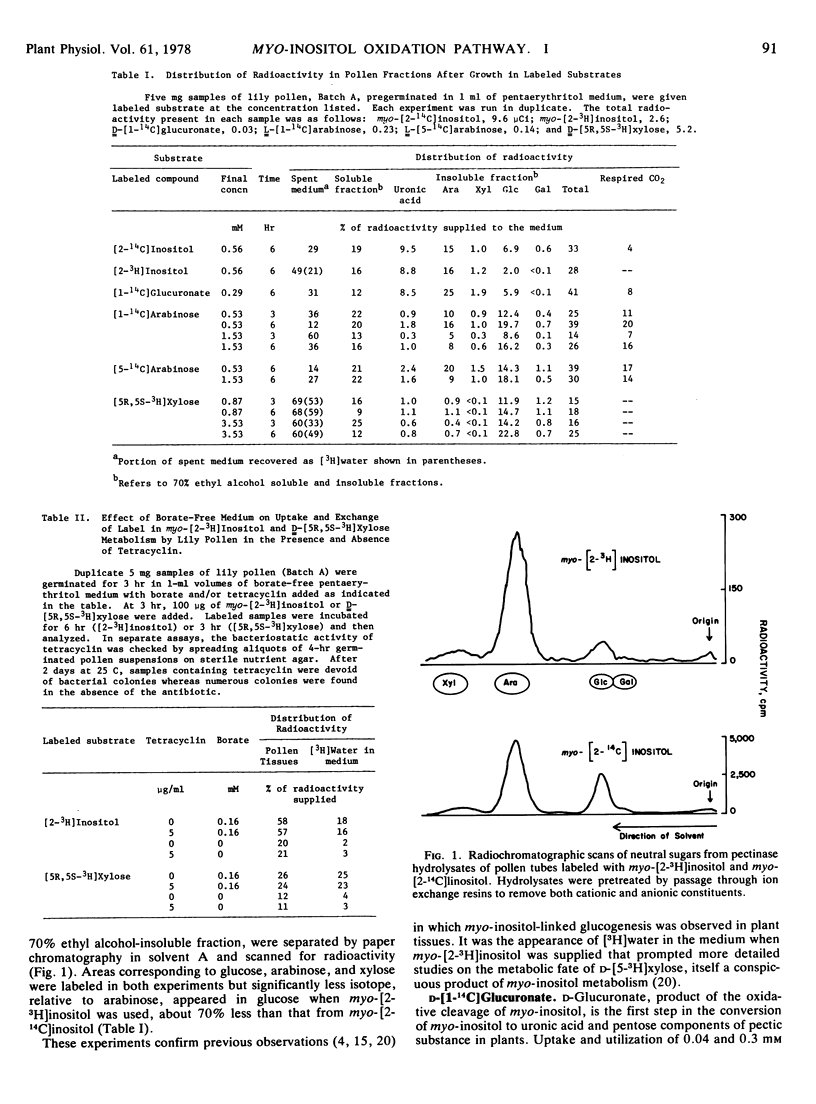
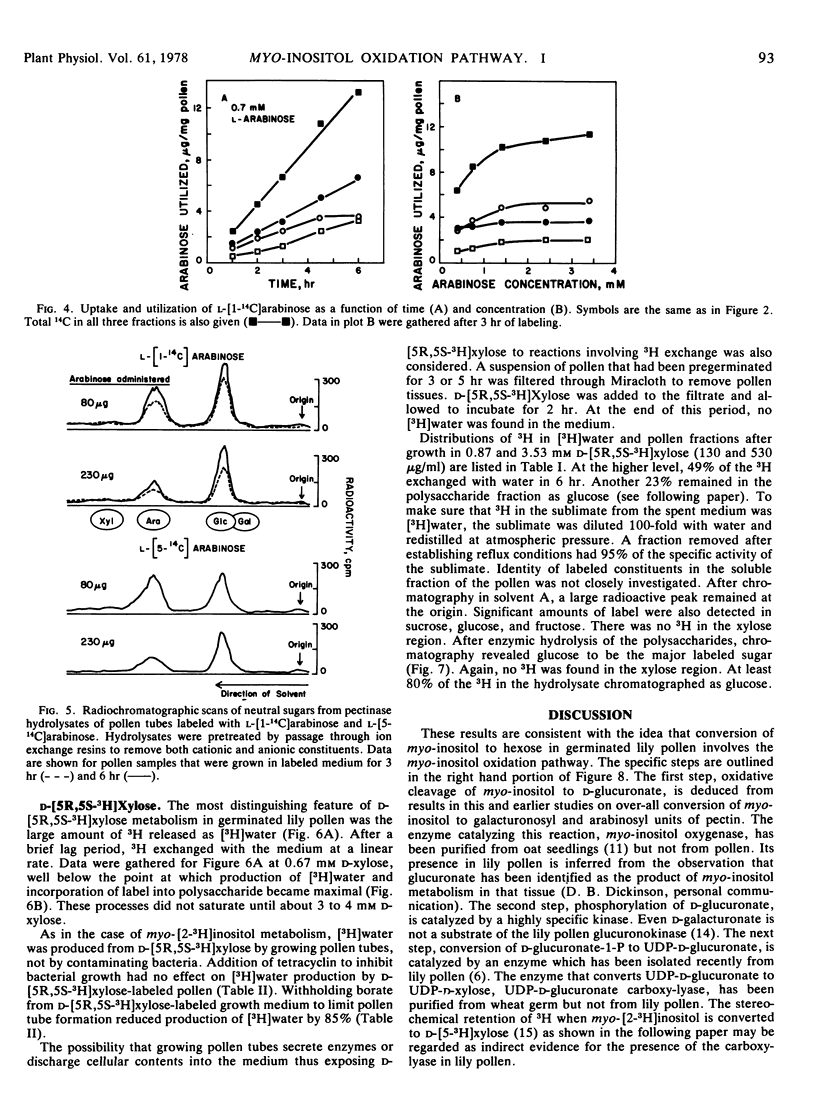
The myo-inositol oxidation pathway was investigated in regard to its role as a source of carbon for products of hexose monophosphate metabolism in germinated pollen of Lilium longiflorum Thunb., cv. Ace. myo-[2-14]Inositol and d-[1-14C]glucuronate had similar distributions of radioactivity, contributing about three times more label to polysaccharide-bound glucose than myo-[2-3H]inositol. In the course of glucogenesis label from the latter appeared as tritiated water in the medium. This exchange could be enhanced by supplying d-[5R,5S-3H]xylose instead of myo-[2-3H]inositol. When the former was administered, [3H]glucose was the only labeled sugar residue found in polysaccharide products. The soluble constituents of d-[5R,5S-3H]xylose-labeled pollen contained no traces of labeled xylose despite massive uptake and utilization.
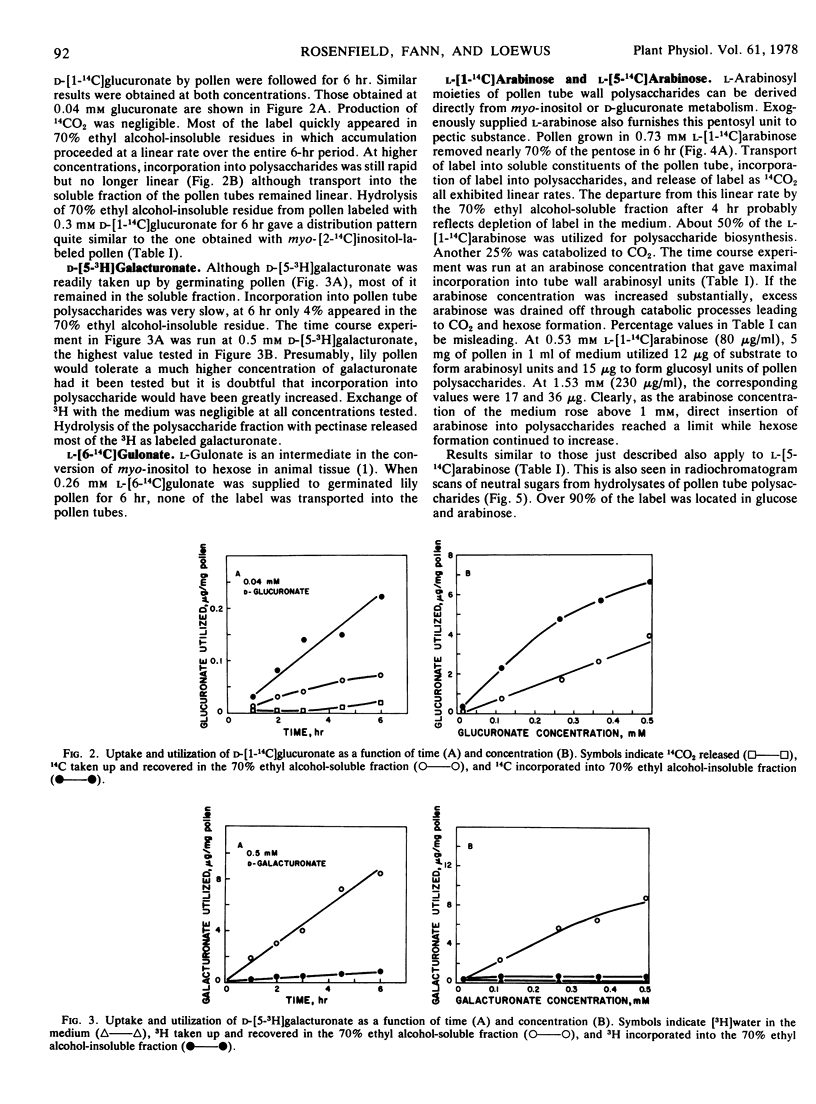
l-[1-14C]- and l-[5-14C]Arabinose produced similar labeling patterns in germinated pollen including incorporation of arabinosyl units into pollen tube polysaccharides and substantial glucogenesis which led to utilization of arabinose for respiration and further incorporation of labeled glucosyl units into pollen tube polysaccharides.
d-[5-3H]Galacturonate was rapidly taken up by germinated pollen but slowly utilized, without conversion to other sugars, for incorporation into pollen tube polysaccharides. l-[6-14C]Gulonate was not taken up by pollen.
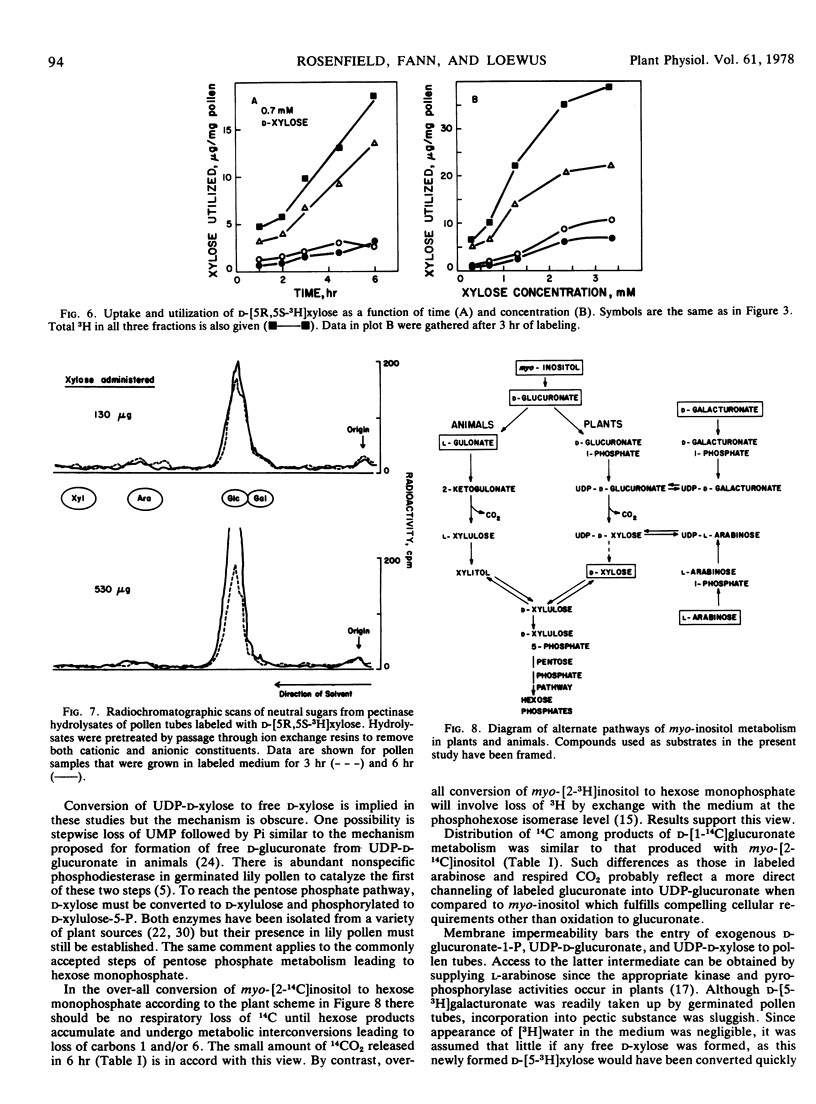
Results strongly support a scheme of conversion from myo-inositol to hexose monophosphate and subsequent products of glucose metabolism that involves the myo-inositol oxidation pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baig M. M., Kelly S., Loewus F. L-ascorbic acid biosynthesis in higher plants from L-gulono-1, 4-lactone and L-galactono-1, 4-lactone. Plant Physiol. 1970 Aug;46(2):277–280. doi: 10.1104/pp.46.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Loewus F. A. myo-Inositol Metabolism in Lilium longiflorum Pollen: Uptake and Incorporation of myo-Inositol-2-H. Plant Physiol. 1977 Apr;59(4):653–657. doi: 10.1104/pp.59.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. D., Dickinson D. B. Properties of uridine diphosphoglucose dehydrogenase from pollen of Lilium longiflorum. Arch Biochem Biophys. 1972 Sep;152(1):53–61. doi: 10.1016/0003-9861(72)90192-0. [DOI] [PubMed] [Google Scholar]
- Dickinson D. B., Hyman D., Gonzales J. W. Isolation of uridine 5'-pyrophosphate glucuronic Acid pyrophosphorylase and its assay using p-pyrophosphate. Plant Physiol. 1977 Jun;59(6):1082–1084. doi: 10.1104/pp.59.6.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English P. D., Dietz M., Albersheim P. Myoinositol kinase: partial purification and identification of product. Science. 1966 Jan 14;151(3707):198–199. doi: 10.1126/science.151.3707.198. [DOI] [PubMed] [Google Scholar]
- FINKLE B. J., KELLY S., LOEWUS F. A. Metabolism of d-[I-14C]- and d-[6-14C] glucuronolactone by the ripening strawberry. Biochim Biophys Acta. 1960 Feb 26;38:332–339. doi: 10.1016/0006-3002(60)91249-x. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem J. 1970 Dec;120(3):479–491. doi: 10.1042/bj1200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller E., Koller F., Hoffmann-Ostenhof O. Myo-inositol oxygenase from oat seedlings. Mol Cell Biochem. 1976 Jan 31;10(1):33–39. doi: 10.1007/BF01731679. [DOI] [PubMed] [Google Scholar]
- LOEWUS F. A. INOSITOL METABOLISM IN PLANTS. II. THE ABSOLUTE CONFIGURATION OF D-XYLOSE-5-T DERIVED METABOLICALLY FROM MYO-INOSITOL-2-T IN THE RIPENING STRAWBERRY. Arch Biochem Biophys. 1964 Jun;105:590–598. doi: 10.1016/0003-9861(64)90055-4. [DOI] [PubMed] [Google Scholar]
- LOEWUS F. A., JANG R. The conversion of C14-labeled sugars to L-ascorbic acid in ripening strawberries. III. Labeling patterns from berries administered pentose-1-C14. J Biol Chem. 1958 May;232(1):521–532. [PubMed] [Google Scholar]
- LOEWUS F. A., KELLY S. The metabolism of p-galacturonic acid and its methyl ester in the detached ripening strawberry. Arch Biochem Biophys. 1961 Dec;95:483–493. doi: 10.1016/0003-9861(61)90180-1. [DOI] [PubMed] [Google Scholar]
- Labarca C., Kroh M., Loewus F. The Composition of Stigmatic Exudate from Lilium longiflorum: Labeling Studies with Myo-inositol, d-Glucose, and l-Proline. Plant Physiol. 1970 Jul;46(1):150–156. doi: 10.1104/pp.46.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Loewus F. The Nutritional Role of Pistil Exudate in Pollen Tube Wall Formation in Lilium longiflorum: II. Production and Utilization of Exudate from Stigma and Stylar Canal. Plant Physiol. 1973 Aug;52(2):87–92. doi: 10.1104/pp.52.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz M. D., Dickinson D. B., Loewus F. A., Loewus M. Partial purification and study of pollen glucuronokinase. Arch Biochem Biophys. 1977 Mar;179(2):559–564. doi: 10.1016/0003-9861(77)90144-8. [DOI] [PubMed] [Google Scholar]
- Loewus F. A., Kelly S., Neufeld E. F. METABOLISM OF MYO-INOSITOL IN PLANTS: CONVERSION TO PECTIN, HEMICELLULOSE, D-XYLOSE, AND SUGAR ACIDS. Proc Natl Acad Sci U S A. 1962 Mar;48(3):421–425. doi: 10.1073/pnas.48.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus M. W., Loewus F. myo-Inositol 1-Phosphate Synthase Inhibition and Control of Uridine Diphosphate-d-glucuronic Acid Biosynthesis in Plants. Plant Physiol. 1974 Sep;54(3):368–371. doi: 10.1104/pp.54.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pubols M. H., Zahnley J. C., Axelrod B. Partial Purification & Properties of Xylose & Ribose Isomerase in Higher Plants. Plant Physiol. 1963 Jul;38(4):457–461. doi: 10.1104/pp.38.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Zahnley J. C., Axelrod B. d-Xylulokinase and d-Ribulokinase in Higher Plants. Plant Physiol. 1965 Mar;40(2):372–378. doi: 10.1104/pp.40.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]


