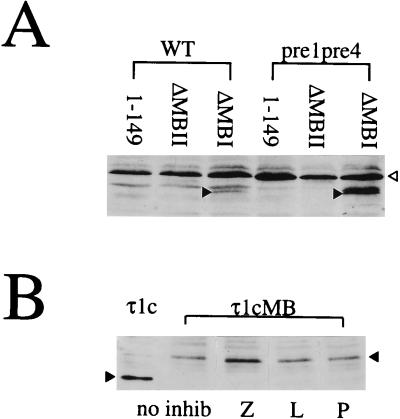
FIG. 5.
Role of the proteasome in myc box-directed degradation. (A) Whole-cell extracts from yeast strains YHI29/W (WT) and YHI29/14 (pre1, pre4) expressing the myc-Pho4 fusion proteins 1–149P, ΔMBIP, and ΔMBIIP were analyzed by Western blotting as described for Fig. 1A. The open arrowhead indicates a nonspecific band recognized by the antibody; the solid arrowheads indicate the ΔMBIP protein band. (B) COS7 cells were transiently transfected with the expression plasmid for τ1cMB. After 42 h of incubation, 10 μM inhibitor (Z-L3 VS [Z], leupeptin [L], or pepstatin [P]) was added. Incubation was continued for a further 4 h. A 30-μg portion of total protein from whole-cell extracts of treated (lanes Z, L, and P) and untreated (lanes no inhib) cells were subjected to SDS-PAGE (20% polyacrylamide) and blotted onto Hybond C-super membrane. The τ1cMB protein was detected with anti GR-DBD antibody (1:3,333 in 1% milk–phosphate-buffered saline–Tween) and secondary sheep anti-mouse IgG-HRP conjugate (1:2,000 in phosphate-buffered saline–Tween). Interaction with the antibodies was visualized by chemiluminescence. An extract from untreated cells producing the τ1c protein was included as a quantification control. Solid arrowheads indicate the positions of the proteins.