Abstract
Background
We aimed to compare the clinical course of patients aged 18–65 years and ≥65years who underwent anterior cervical discectomy and fusion (ACDF) or corpectomy for ventrally located CSEA.
Methods
Clinical and imaging data were retrospectively collected from the institutional database between September 2005 and December 2021.
Results
A total of 35 and 26 patients aged 18–64 and ≥ 65 years, respectively who were diagnosed with ventrally located CSEA were included. The overall mean age was 63.9 ± 3.2 years, with a predominance of the male sex (n = 43/61, 70.5%). Patients aged ≥65 years presented with significantly higher rates of comorbidities (10.3 ± 2.8), as indicated by the CCI, than their younger counterparts (18–64 years: 6.2 ± 2.6; p < 0.001). No differences in the surgical approach or characteristics were observed among the groups. Notably, patients aged ≥65 years had a significantly longer intensive care unit as well as overall hospital stay. In-hospital and 90-day mortality were similar across both groups. Following both types of surgery, a significant improvement was observed in the blood infection parameters and neurological status at discharge compared with the baseline measurements. Older age, higher rates of comorbidities, and higher grades of disability were significant predictors for mortality.
Conclusions
Emergency surgical evacuation should be undertaken for CSEA in the presence of acute neurological deterioration regardless of the age. Factors, such as age, comorbidities, and neurological status on admission appear to be important predictors of disease outcomes. However, the risk profile of younger patients should not be underestimated.
Keywords: Cervical epidural abscess, Age, Motor deficit, Corpectomy, ACDF
Abbreviations list
- ACDF
anterior cervical discectomy and fusion
- CSEA
cervical spinal epidural abscess
- SEA
spinal epidural abscess
- IVDU
intravenous drug abuse
- CT
computed tomography
- CCI
age-adjusted Charlson comorbidity index
- ASA
American Society of Anesthesiologists Physical Status Classification System
- LOS
length of stay
- ICU
intensive care unit
- mJOA
modified Japanese Orthopedic Association
- CRP
C-reactive protein
- COPD
chronic obstructive pulmonary disease
- MS
motor score
1. Introduction
Spinal epidural abscess (SEA) is a rare spinal infection that occurs between the thecal sac of the spinal cord, spinal ligaments, and vertebrae. There has been increasing incidence of SEA with recent estimates ranging from 2 to 12.5 cases per 10,000 hospital admissions.1, 2, 3 The potential contributing factors to this increase in incidence may be due to the high prevalence of intravenous drug abuse (IVDU) and steep growth of the aging population, who often present with a weakened immune system and are therefore more susceptible to spinal infections.3, 4, 5 Cervical SEA (CSEA) is an even rarer form of SEA, compared to thoracic or lumbar SEA. CSEA accounts for approximately 14% of all SEAs.6 Unlike the highly variable presentation and clinical course of SEA, which can complicate diagnosis, patients with CSEA often present with acute neurological deterioration.1 Therefore, immediate initiation of treatment is mandatory. Although the underlying mechanisms contributing to the steep neurological decrease are not elucidated, the most widely accepted theory suggests that the smaller epidural space is less permissive of abscess and inflammation.4,7
Surgical intervention is currently considered the gold standard for the management of CSEA in patients presenting with new neurological deficits; however, robust evidence on this topic is still lacking. Considering the rapid growth of the aging population and the increased incidence of the disease among this age group, surgeons are often reluctant in suggesting surgical intervention. Previous studies have suggested that surgical procedures can produce sufficient outcomes with a good safety profile even in this age group.8, 9, 10 Various surgical procedures have been proposed, such as anterior cervical discectomy and fusion for ventrally located CSEA, corpectomy when osteomyelitis is present, or combined procedures.11
A significant gap exists in the literature concerning comparative studies focusing on the treatment outcomes of young and older patients with CSEA. Considering this lack of clinical evidence, we aimed to compare and assess the clinical course, morbidity, and mortality in patients with CSEA aged 18–64 years and ≥65 years who underwent emergency surgery for CSEA management.
2. Methods
2.1. Study design
All the data (imaging and clinical) were retrospectively collected from our institution's database between September 2005 and December 2020. This study was approved by the local ethics committee of our institution (no. 880/2021) and was conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived owing to the retrospective nature of the study. Patients aged ≥18 years with CSEA were enrolled consecutively. The diagnosis was based on magnetic resonance imaging. Spine stability was examined using computed tomography. The exclusion criteria were: age <18 years, concurrent intracranial or cervical pathology, and unavailable data.
The exclusion criteria were spinal instability, including bony deconstruction resulting in kyphosis or subluxation of the vertebral column, vertebral collapse of more than 50% or bone necrosis, and complete loss of disc height.
2.2. Electronic records
Electronic medical records were assessed to obtain patient demographics, comorbidities, American Society of Anesthesiologists scores, duration of surgery, number of treated spinal levels, peri- and postoperative complications, hospital length of stay, intensive care unit (ICU) stay, readmission, reoperation, and mortality. Comorbidities present before surgery were assessed using the age-adjusted Charlson comorbidity index (CCI).12,13 The CCI was calculated for each patient and classified as no (CCI = 0), minimal (CCI = 1 or 2), moderate (CCI = 3–5), or severe (CCI >5) comorbidity. The pretreatment neurological condition was assessed using the modified Japanese Orthopedic Association (mJOA) score for cervical myelopathy.14 Post-treatment mJOA data were obtained from the most recent documented clinical encounter. Routine clinical and radiological follow-up examinations were performed before discharge and 3 months after surgery. The final follow-up ranged between 3 and 62 months postoperatively. Standard radiographs in the anteroposterior and lateral views were obtained to evaluate the screw position and fusion rate.
2.3. Surgical procedures
Patients were allocated to one of the following age groups: 1) 18–64 years, and 2) ≥65 years. Anterior cervical discectomy and fusion (ACDF) (insert Fig. 1) or corpectomy (insert Fig. 2) were performed. Decision-making was guided by the presenting neurological status, concomitant underlying pathologies, extent of the pathology, and discretion of an experienced treatment team consisting of neurosurgeons, neuroradiologists, and anesthesiologists. The final decision for ACDF or corpectomy with plate fixation was made according to the surgeons’ preference. In line with our institutional treatment protocols, blood samples or intraoperative cultures were collected before administering intravenous (IV) antibiotics. Thereafter, IV antibiotics were immediately initiated. After identifying the bacterial specimens, the choice of IV antibiotics was adapted to reflect the antibiogram results.
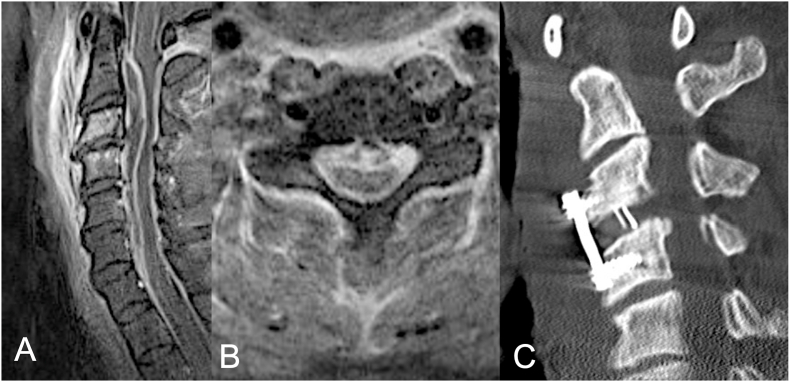
Fig. 1.
Postcontrast sagittal (A) and axial (B) T2-weighted magnetic imaging of ventral cervical epidural abscess of C2 and C6 of a 32-year-old male patient with intravenous drug abuse presenting with progressive tetraparesis. (C) Postoperative computed tomography (CT) of the cervical spine depicting the cervical anterior cervical discectomy and fusion extending from level C3 to C4 with interbody graft and plating seen at C3–C4. The ventral epidural abscess was evacuated via catheter-based drainage.
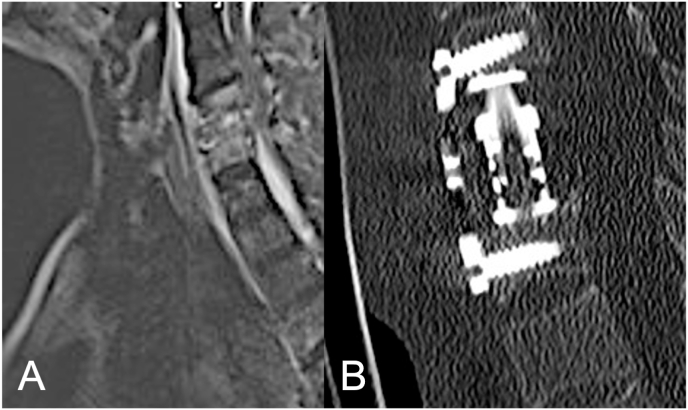
Fig. 2.
Postcontrast sagittal (A) and axial (B) T2-weighted magnetic imaging of ventral cervical epidural abscess of C3 and C4 and early endplate desctruction of C3 and C4 of an 21-year-old female patient with IVDA presenting with progressive weakness of low extremities. (C) Postoperative computed tomography (CT) of corpectomy with placement of a ventral at C3–C4.
2.4. Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables are presented as means ± standard deviations and were verified as normally distributed using the Shapiro–Wilk test. Baseline and surgical characteristics were compared by group-wise comparison using independent t-tests for continuous variables and chi-squared tests for categorical variables. The Wilcoxon rank test was used to evaluate the changes in the C-reactive protein (CRP) level, leukocyte count, and neurological status (mJOA) of each group at discharge. A binary logistic regression analysis was performed to assess potential confounders for mortality. Statistical significance was set at a p-value of 0.05.
3. Results
Over a period of 16 years, 35 patients aged 18–64 years and 26 aged ≥65 years who were diagnosed with ventrally located CSEA were included. The overall mean age was 63.9 ± 3.2 years, with a predominance of the male sex (n = 43/61, 70.5%). Patients ≥65 years presented with significantly higher rates of comorbidities (10.3 ± 2.8), as indicated by CCI, than their younger counterparts (18–64 years: 6.2 ± 2.6; p < 0.001). The prevalence rates of cardiovascular diseases, peripheral vascular diseases, diabetes mellitus type II, chronic obstructive pulmonary disease (COPD), dementia, and renal and liver failure were significantly higher in the older age group. Both IV drug and alcohol abuse were highly prevalent in the 18–64-year-old group. The rates of laboratory infection parameters were similarly high in both the groups, while older patients presented with higher grades of disability (65–79 years: 10.2 ± 3.7 vs. ≥65 years: 8.1 ± 3.3; p = 0.012), as evidenced by the mJOA score. The baseline characteristics are depicted in Table 1.
Table 1.
Baseline patient characteristics.
| 18–64 y n = 35 | ≥65 y n = 26 | p-value | |
|---|---|---|---|
| Age, y (mean, SD) | 54.9 (8.0) | 72.8 (4.2) | <0.001 |
| Sex (n, %) | 0.343 | ||
| Male | 23 (65.7) | 20 (76.9) | |
| Female | 12 (34.3) | 6 (23.1) | |
| BMI, kg/m2 (mean, SD) | 28.6 (5.5) | 27.4 (5.7) | 0.938 |
| Comorbidities | |||
| Age-adjusted CCI score (mean, SD) | 6.2 (2.6) | 10.3 (2.8) | <0.001 |
| Arterial hypertension (n, %) | 15 (42.9) | 19 (73.1) | 0.019 |
| Myocardial infarction (n, %) | 8 (22.9) | 20 (76.9) | <0.001 |
| Coronary heart disease (n, %) | 8 (22.9) | 18 (69.2) | <0.001 |
| Atrial fibrillation (n, %) | 0 (0.0) | 9 (34.6) | <0.001 |
| Heart failure (n, %) | 1 (2.9) | 6 (23.1) | 0.014 |
| Peripheral vascular disease | 3 (8.6) | 7 (26.9) | 0.056 |
| COPD (n, %) | 4 (11.4) | 10 (38.5) | 0.013 |
| Diabetes mellitus Type II (n, %) | 6 (17.1) | 12 (46.2) | 0.014 |
| Renal failure (n, %) | 2 (5.7) | 21 (80.8) | 0.002 |
| Liver disease (n, %) | 7 (20.0) | 8 (30.8) | 0.334 |
| Gastrointestinmalignnacy.al ulcer (n, %) | 2 (5.7) | 7 (26.9) | 0.021 |
| TIA/stroke (n, %) | 0 (0.0) | 5 (19.2) | 0.007 |
| Malignancy (n, %) | 4 (11.4) | 11 (42.2) | 0.006 |
| Dementia (n, %) | 0 (0.0) | 7 (26.9) | 0.001 |
| Alcohol abuse (n, %) | 10 (28.6) | 8 (30.8) | 0.852 |
| Drug abuse (n, %) | 5 (14.3) | 1 (3.8) | 0.176 |
| Previous spinal surgery (n, %) | 1 (2.9) | 6 (23.1) | 0.065 |
| ASA class (n, %) | 0.146 | ||
| I | 3 (8.6) | 4 (15.4) | |
| II | 10 (28.6) | 17 (65.4) | |
| III | 21 (60.0) | 4 (15.4) | |
| IV | 0 (0.0) | 5 (3.8) | |
| V | |||
| CRP level, mg/L (mean, SD) | 122.4 (6.5) | 145.8 (7.1) | 0.855 |
| Leukocytes, count/L (mean, SD) | 12.8 (2.1) | 14.5 (8.2) | 0.652 |
| Preoperative mJOAj score (mean, SD) | 10.2 (3.7) | 8.1 (3.3) | 0.012 |
N, group size; n, number of patients; ASA, American Society of Anesthesiologists; BMI, body mass index; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; mJOA, modified Japanese Orthopedic Association; SD, standard deviation; TIA, transient ischemic attack.
4. Surgical procedures and clinical course
As shown in Table 2, no differences in the surgical approach or characteristics were observed among the groups. Notably, patients aged ≥65 years had a significantly longer ICU as well as overall hospital stay. In-hospital and 90-day mortality were similar across both groups (18–64 years: n = 1, 2.9%, 90-day mortality n = 5 14.3% vs. ≥65 years: n = 1, 3.8% 90-day mortality n = 4, 15.4%; p > 0.05), The laboratory parameter levels indicative of infection and motor impairment significantly improved in a steeper manner in younger patients. The overall mean follow-up period was 45.2 ± 11.1 months, and no reoperations due to secondary instability or deaths occurred. According to the radiographs, no cases of screw loosening or displacement were observed. After surgery, a significant decrease in the CRP levels and leukocyte count and amelioration of mJOA were observed in all the groups at discharge compared to the baseline levels, as displayed in Table 3.
Table 2.
Comparison of the surgical characteristics and clinical course between the groups.
| 18–64 y n = 35 | ≥65 y n = 26 | p-value | |
|---|---|---|---|
| Surgical approaches (n,%) | 0.912 | ||
| ACDF | 18 (51.4) | 13 (50.0) | |
| Corpectomy | 17 (48.6) | 13 (50.0) | |
| Intraoperative blood loss (ml) | 340 (120.1) | 400 (135.8) | 0.687 |
| Surgical duration, min | 176.7 (110.5) | 175.2 (83.1) | 0.586 |
| No. of levels decompressed/fused | 1.3 (0.5) | 1.7 (0.6) | 0.058 |
| Hospital stay, days | 14.7 (10.8) | 18.8 (9.1) | 0.008 |
| ICU stay, days | 5.9 (3.2) | 7.8 (1.2) | 0.051 |
| Mortality | |||
| In-hospital (n, %) | 1 (2.9) | 1 (3.8) | 0.830 |
| 90-day (n, %) | 5 (14.3) | 4 (15.4) | 0.905 |
| 30-day readmission, days | 1.8 (1.1) | 1.9 (0.6) | 0.445 |
| Post CRP | 42.7 (11.2) | 98.8 (22.2) | 0.004 |
| Post leukocytes | 9.3 (3.4) | 10.3 (3.9) | 0.217 |
| Post mJOAe | 13.4 (3.5) | 10.1 (2.8) | 0.003 |
Except where otherwise indicated, quantities are mean (SD); bold = significant difference; Post, after surgery; Delta, difference between pre-and postsurgical values. ACDF, anterior cervical discectomy and fusion; CRP, C-reactive protein; ICU, intensive care unit; mJOA, modified Japanese Orthopedic Association.
Table 3.
Occurrence of adverse events.
| 18–64 y n = 35 | ≥65 y n = 26 | p-value | |
|---|---|---|---|
| Deep wound infection | 1 (2.9) | 4 (15.4) | 0.330 |
| Acute respiratory failure | 0 (0.0) | 1 (3.8) | 0.831 |
| Acute heart failure | 2 (5.7) | 3 (11.5) | 0.412 |
| Acute renal failure | 3 (8.6) | 4 (15.4) | 0.409 |
| Pneumonia | 2 (5.7) | 8 (30.8) | 0.008 |
| Dysphagia | 1 (2.9) | 3 (11.5) | 0.176 |
| Urinary tract infection | 2 (5.7) | 1 (3.8) | 0.764 |
All data indicate number of patients (%). bold = significant difference.
Among the patients with ventrally located cervical epidural abscesses, six cases were associated with drug abuse. The age distribution of these patients revealed a significant disparity; one was over the age of 80, while the remaining five were considerably younger with a mean age of 26.2 years (SD = 1.2). These younger individuals were intravenous drug users and presented with clinical signs indicative of systemic inflammation and neurological impairment. Laboratory findings for this subgroup demonstrated elevated mean C-reactive protein (CRP) levels at 115 mg/L (SD = 1.2) and mean leukocyte counts of 12 x 10^9/L (SD = 1.2), with clinical presentations of paraparesis affecting the lower extremities.
The surgical intervention for these five younger patients consisted of corpectomy, which is aligned with the management strategy for severe cases presenting with significant neurological deficits and/or structural instability.
The sole patient above the age of 80 had a history of chronic drug abuse, further complicated by renal insufficiency necessitating dialysis. This individual presented with an inability to ambulate and more severe inflammatory markers, with a CRP level of 157 mg/L and leukocyte count of 14.1 x 10^9/L. The chosen surgical approach for this older patient was anterior cervical discectomy and fusion (ACDF), reflecting a tailored approach considering the patient's age, comorbid conditions, and the surgical risk profile.
5. Complications and risk factors for mortality
Pneumonia occurred at a significantly higher rate in patients ≥65 years (n = 8, 30.8%) than in younger patients (n = 2, 5.7%; p = 0.008). A detailed description of complications is provided in Table 4. The prevalence of infection with Staphylococcus aureus was high in both the groups (18–64 years: 65.4%, ≥65 years: 55.8%), either in the blood or intraoperative samples. Binary logistic regression analysis revealed that older age (≥65 years), presence of comorbidities, and poor preoperative neurological condition were significant predictors of mortality, while the surgical approach itself and level of infection were not (Table 5).
Table 4.
Comparison between the baseline (before surgery) and at the time of discharge.
| 18–64y Baseline n = 35 |
18–64y Discharge n = 35 |
p-value | ≥65 y Baseline n = 26 |
≥65 y Discharge n = 26 |
p-value | |
|---|---|---|---|---|---|---|
| CRP | 122.4 (6.5) | 42.7 (11.2) | <0.001 | 145.8 (7.1) | 98.8 (22.2) | 0.021 |
| Leukocytes | 12.8 (2.1) | 9.3 (3.4) | 0.007 | 14.5 (8.2) | 10.3 (3.9) | 0.019 |
| mJOA | 10.2 (3.7) | 13.4 (3.5) | <0.001 | 8.1 (3.3) | 10.1 (2.8) | <0.001 |
All data are mean (standard deviation). bold = significant difference.
CRP, C-reactive protein; mJOA, modified Japanese Orthopedic Association.
Table 5.
Risk factors associated with mortality.
| Risk factor | OR (95% CI) | p-value |
|---|---|---|
| Age >65 years | 1.2 (1.1–3.4) | 0.001 |
| Age-adjusted CCI score | 1.8 (1.1–5.2) | 0.002 |
| Preoperative MS | 1.7 (1.1–2.4) | 0.032 |
| Preoperative CRP | 1.1 (1.0–1.8) | 0.068 |
| Duration of surgery | 1.0 (0.9–1.3) | 0.786 |
| Number of levels decompressed | 0.5 (0.1–1.0) | 0.889 |
| ACDFb | 1.3 (1.1–2.1) | 0.201 |
CCI, Charlson Comorbidity Index; CI, confidence interval; ICU, intensive care unit; MS, motor score of the American Spinal Injury Association grading system; OR, odds ratio; ACDF: anterior cervical decompression and fusion.
corpectomy.
6. Discussion
CSEA is important because patients immediately present with acute neurological deterioration with signs of infection that requires prompt initiation of treatment.2,15 While surgical management of CSEA is the standard approach of treatment, decision-making is performed on a case-to-case basis to ensure a positive outcome, taking into consideration factors such as age, comorbidity rates, and location of the abscess relative to the dural sack. However, to date, systematic analyses on the surgical treatment of young and older patients, as well as the potential risk factors associated with their therapy are lacking.
To the best of our knowledge, this study is the first to compare and assess the outcomes of young (18–64 years) and older (≥65 years) patients who underwent surgery for ventrally located CSEA. This study employed ACDF with plate or corporectomy as the surgical approach. The results revealed that older patients presented with significantly higher rates of comorbidities, particularly higher rates of cardiovascular diseases, COPD, renal and liver failure, diabetes mellitus type II, and malignancy. No significant difference was noted concerning the infection levels in the blood or the neurological condition; however, it is important to highlight that younger patients had a steeper recovery period compared to their older counterparts. As expected, older patients warranted significantly longer hospital stay and were even admitted in the ICU. However, hospital and 90-day mortality were similar in both the groups. Both the groups had similar recovery rates concerning blood infection and neurological deficits. Notably, age, comorbidities, and preoperative neurological condition were unique risk factors for mortality, while the surgery itself was not.
7. Review of literature
Owing to the increasing life expectancy, the proportion of elderly patients continues to grow. Thus, surgeons frequently face challenges in choosing the treatment modality for such patients, which is formidable owing to their poor baseline reserve. Particularly, older patients suffering from CSEA represent a unique subset since many compounding factors should be considered before opting for surgical management. In the retrospective study of Shweikeh et al on 16 patients suffering from CSEA with a mean age of 57.9 years, they found vascular diseases to be the most prevalent risk factor for the development of CSEA, followed by diabetes mellitus, renal failure, malignancy, and drug abuse.16 Similarly, the study by Adowga et al also demonstrated multiple medical problems as predisposing risk factors for CSEA with diabetes mellitus and end stage renal failure inducing the highest risks for affliction with this devastating disease.17 In the retrospective analysis of Kim et al on 16 patients aged ≥65 years, they reported diabetes mellitus as the most prevalent underlying disease followed by cardiovascular illnesses.18 In the largest meta-analysis on 915 patients with SEA, diabetes mellitus was demonstrated as a paramount factor for immunocompromised patients since inadequately treated blood glucose is linked to reduced cellular immunity; thus, a substantial decrease of chemotaxis, phagocytosis, and bacterial activity of neutrophilic granulocytes takes place.15 Notably, the distinct difference between the aforementioned studies and the present analysis is that our study includes a comparison between young and old patients and the potential differences of these age groups, which were not investigated in previous studies. In the present study, patients were expected to suffer from significantly higher rates of comorbidities as denoted by a comorbidity channel index of 10.3 compared to their younger counterparts. Consistent with the results of previous studies, we reported a similar prevalence of comorbidities with diabetes mellitus, renal failure, and cardiovascular diseases being the most prevalent. IV drug and alcohol abuse were more common among younger patients. We consider that a meticulous preoperative evaluation of patient profile is critical not only for therapeutic decision-making but also for predicting and assessing outcomes.
Interestingly, no significant difference was observed between the groups in terms of blood infection levels at baseline; nevertheless, a steep decrease over half of the initial values was observed solely in the younger patients. In older patients, the infection levels improved but at a significantly slower pace. This may be attributed to a decreased immune response owing to poor baseline history. For example, patients with chronic renal failure present with high levels of blood inflammatory markers and due to the necessity of renal protection, antibiotic dosage is decreased. Hence, a delayed reduction in the number of T cells and cytokine levels is observed as compared to younger patients who receive complete adult dosing.19
In this study, the patients, regardless of age, presented with an acute neurological decline; thus, emergency surgical therapy was performed, even in older patients. In a retrospective study on 59 patients with a mean age of 59 years, who underwent surgery in less than 24 h after admission owing to the presence of new neurological deficits, all the patients experienced neurological improvement of at least 1 point according to the ASIA scale and no neurological progress of deficits was observed.20 Similarly, another study found that emergent surgical decompression of CSEA resulted in an improvement or halting of further neurological deterioration in 40 patients with a mean age of 53 years.21 In a retrospective series of 62 patients by Alton et al, 70% of those who underwent pharmacologic intervention had not recovered and deteriorated further neurologically; thus, these patients underwent delayed surgery. Although some motor function was preserved, delayed surgery resulted in a substantial net loss of motor function in nearly 16 points as measured by the motor score (MS) of the ASIA impairment scale, while patients undergoing early surgery improved by more than 11 points in their motor capability as shown by the MS. Hence, the study concluded that early surgery could be the key tool in maintaining or even improving patient neurological condition.1 The pathogenic factor for neurological symptoms may be attributed to the mechanical compression on the dural sac caused by the SEA, presumably causing vascular compression with secondary hypoxia.15 Since the epidural space in the cervical spine is much smaller compared to the lumbar or thoracic one, severe deficits are expected. Consequently, a combination of ischemic events with progressive compression seems to be the major contributory factor for patients with neurological deterioration; therefore, prompt surgical decompression might be the benchmark of SEA in the presence of acute neurological deterioration.
The optimal surgical technique in CSEA remains debatable. In the present series, patients presented with ventrally located CSEA; thus, anterior approach was considered for its evacuation. The same surgical technique (ACDF and corpectomy) was performed regardless of the patient's age. In a recent study on the clinical and radiological outcomes in 30 patients with CSEA undergoing surgery, Shousha et al described 13 cases of ACDF and four cases of corpectomy.22 Good fusion and acceptable restoration of lordosis were achieved with both surgical techniques, although two cases from the corpectomy group needed further operation with additional posterior fixation owing to graft dislocation. Similar to these findings, Ghorbrial et al, in their series of 40 patients with CSEA, reported four cases of corpectomy involving one or two levels, with good fusion rates. However, one case (25.0%) presented with a high-grade of pseudarthrosis at the eighth month follow-up, which required posterior stabilization.21 Likewise, in our study, two older patients received additional posterior instrumentation owing to dislocation of the pseudoarthrosis following corpectomy, while none of the patients who underwent ACDF required further surgery. Patients <65 years did not require revision surgery over a 2-year follow up. Muzii et al described nine patients with CSEA who underwent one- or two-level ACDF with concomitant administration of antibiotics. None of the patients underwent revision surgery; fusion occurred in all the cases.
It is noteworthy to mention that these previous studies did not stratify patients according to age; hence, these findings cannot be completely applied to the decision-making process for surgical interventions. According to our findings, older patients had significantly longer hospital stays (and were even admitted in the ICU) compared to their younger counterparts. The most plausible explanation for this is that the older population has decreased baseline reserve and immune response, which results in a greater possibility of postoperative complications. Pneumonia was the most prevalent complication among the older patients requiring prolonged intensive care. Postoperative pneumonia is the third most common complication of all the surgical procedures associated with increased patient morbidity and mortality.23 Regardless of age, the mortality ranged between 14 and 15.0% indicating that age is not the primary factor for not performing surgery, rather a collective understanding of patient profile should unambiguously guide the decision-making process. Various literature support these findings, which have reported similar mortality rates for anterior procedures (ACDF and corpectomy) with a breadth of 11–15%.15,16,22
To deeply understand the potential impact of the factors on mortality, we performed a regression analysis to examine various factors associated with patient mortality. The results demonstrated that age ≥65 years, higher rates of comorbidities, and reduced neurological condition were significant predictors of patient mortality, while complications (such as pneumonia), or surgical technique, or duration of surgery were not. While these findings may seem counterintuitive, since one might expect surgery or postoperative complications to be risk factors for patient mortality, special attention should be focused on these three factors before proceeding with the operation and when communicating with patients and their relatives concerning the clinical course and patient outcomes. These findings are also supported by previous studies, which have identified several risk factors for mortality, such as greater age with a relative risk (RR) increasing from 1.0 for age 50–59 years to 9.5 for age >70 years, higher grades of dependency (RR 4.4), operative duration (RR 2.2 for 150–199 min), two or more operation levels (RR 1.7) as well as the presence of diabetes mellitus (RR 2.4–2.8).23
8. Limitations
Our study was the first to examine the outcomes of young (18–64 years) patients and those undergoing surgery for two-level CSEA aged ≥65 years. However, this study has certain limitations. First, we examined a relatively small cohort of patients. Nevertheless, since CSEA is rare and most of the evidence is based on small-scale retrospective studies or case reports, we believe that our study produces a real world picture of the disease and most importantly could be applied as a tool to guide decision making based on the patient's age. Second, the minimum follow-up period of 12 months was relatively short. By gathering long-term data, other relevant findings could have been revealed. Third, since this was a retrospective study, selection bias could not have been eliminated. Fourth, The small number of inclusions precluded us from capturing the full spectrum of clinical scenarios, particularly those challenging cases where the etiology remains elusive. We understand that this is an important aspect of the clinical management of epidural abscesses and warrants further investigation. Hence, we propose that future prospective studies with larger sample sizes are required to explore this issue in greater depth. Such studies would help to determine the frequency of unknown etiologies and develop management strategies that could potentially improve outcomes in this subset of patients.
9. Conclusions
No applicable guidelines for CSEA exist owing to the heterogeneous and often comorbid patient population, and the availability of a wide variety of treatment options. However, management remains challenging. Thus, emergency surgical evacuation should be undertaken for CSEA in the presence of acute neurological deterioration regardless of the patient's age. Factors, such as age, comorbidities, and neurological status on admission appear to be important predictors of the disease outcomes. However, the risk profile of younger patients could not be underestimated, since drug and alcohol abuse, which is prevalent in this age group, contributes to the compromise of their immune system.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Consent to participate
The requirement for informed consent was waived on account of the retrospective nature of the study.
CRediT authorship contribution statement
Pavlina Lenga: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Gelo Gülec: Methodology, Data curation. Karl Kiening: Investigation, Conceptualization. Andreas W. Unterberg: Writing – review & editing. Basem Ishak: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Alton T.B., Patel A.R., Bransford R.J., Bellabarba C., Lee M.J., Chapman J.R. Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses. Spine J Off J North Am Spine Soc. 2015;15(1):10–17. doi: 10.1016/j.spinee.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Arko L., Quach E., Nguyen V., Chang D., Sukul V., Kim B.S. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus. 2014;37(2):E4. doi: 10.3171/2014.6.FOCUS14127. [DOI] [PubMed] [Google Scholar]
- 3.Patel A.R., Alton T.B., Bransford R.J., Lee M.J., Bellabarba C.B., Chapman J.R. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J Off J North Am Spine Soc. 2014;14(2):326–330. doi: 10.1016/j.spinee.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 4.Darouiche R.O. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012–2020. doi: 10.1056/NEJMra055111. [DOI] [PubMed] [Google Scholar]
- 5.Pereira C.E., Lynch J.C. Spinal epidural abscess: an analysis of 24 cases. Surg Neurol. 2005;63(1):S26–S29. doi: 10.1016/j.surneu.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Giuffrida S., Chiaramonte I., Saponara R., et al. Cervical epidural abscess: serial MRI study. J Neurosurg Sci. 1997;41(2):219–223. [PubMed] [Google Scholar]
- 7.Turner A., Zhao L., Gauthier P., Chen S., Roffey D.M., Wai E.K. Management of cervical spine epidural abscess: a systematic review. Ther Adv Infect Dis. 2019;6 doi: 10.1177/2049936119863940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenga P., Gülec G., Bajwa A.A., et al. Decompression only versus fusion in octogenarians with spinal epidural abscesses: early complications, clinical and radiological outcome with 2-year follow-up. Neurosurg Rev. May 10, 2022 doi: 10.1007/s10143-022-01805-4. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenga P., Gülec G., Bajwa A.A., et al. Surgical management of spinal epidural abscess in elderly patients: a comparative analysis between patients 65-79 Years and ≥80 Years with 3-year follow-up. World Neurosurg. August 28, 2022;22:1878–8750. doi: 10.1016/j.wneu.2022.08.095. Published online. 01206-2. [DOI] [PubMed] [Google Scholar]
- 10.P L., G G., Aa B., et al. ACDF versus corpectomy in octogenarians with cervical epidural abscess: early complications and outcomes with 2 years of follow-up. Acta Neurochir. January 11, 2023 doi: 10.1007/s00701-023-05488-8. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stricsek G., Iorio J., Mosley Y., et al. Etiology and surgical management of cervical spinal epidural abscess (SEA):: a systematic review. Glob Spine J. 2018;8(4):59S–67S. doi: 10.1177/2192568218772048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groot V., Beckerman H., Lankhorst G.J., Bouter L.M. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 13.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Benzel E.C., Lancon J., Kesterson L., Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4(3):286–295. doi: 10.1097/00002517-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Reihsaus E., Waldbaur H., Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;23(4):175–204. doi: 10.1007/pl00011954. discussion 205. [DOI] [PubMed] [Google Scholar]
- 16.Shweikeh F., Saeed K., Bukavina L., Zyck S., Drazin D., Steinmetz M.P. An institutional series and contemporary review of bacterial spinal epidural abscess: current status and future directions. Neurosurg Focus. 2014;37(2):E9. doi: 10.3171/2014.6.FOCUS14146. [DOI] [PubMed] [Google Scholar]
- 17.Adogwa O., Karikari I.O., Carr K.R., et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15-year institutional perspective and review of the literature: clinical article. J Neurosurg Spine. 2014;20(3):344–349. doi: 10.3171/2013.11.SPINE13527. [DOI] [PubMed] [Google Scholar]
- 18.Kim J.H., Lee E.H., Kim J., Kim C. Clinical outcome of spinal epidural abscess in elderly patients. The Nerve. 2020;6(2):42–49. doi: 10.21129/nerve.2020.6.2.42. [DOI] [Google Scholar]
- 19.Lu K.L., Huang W.H., Lu Y.A., et al. Identifying risk groups of infectious spondylitis in patients with end-stage renal disease under hemodialysis: a propensity score-matched case-control study. BMC Nephrol. 2019;20(1):323. doi: 10.1186/s12882-019-1504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghobrial G.M., Franco D., Theofanis T., et al. Cervical spondylodiscitis: presentation, timing, and surgical management in 59 patients. World Neurosurg. 2017;103:664–670. doi: 10.1016/j.wneu.2017.04.119. [DOI] [PubMed] [Google Scholar]
- 21.Ghobrial G.M., Viereck M.J., Margiotta P.J., et al. Surgical management in 40 consecutive patients with cervical spinal epidural abscesses: Shifting toward Circumferential treatment. Spine. 2015;40(17):E949. doi: 10.1097/BRS.0000000000000942. [DOI] [PubMed] [Google Scholar]
- 22.Shousha M., Boehm H. Surgical treatment of cervical spondylodiscitis: a review of 30 consecutive patients. Spine. 2012;37(1):E30–E36. doi: 10.1097/BRS.0b013e31821bfdb2. [DOI] [PubMed] [Google Scholar]
- 23.Chughtai M., Gwam C.U., Mohamed N., et al. The Epidemiology and risk factors for postoperative pneumonia. J Clin Med Res. 2017;9(6):466–475. doi: 10.14740/jocmr3002w. [DOI] [PMC free article] [PubMed] [Google Scholar]