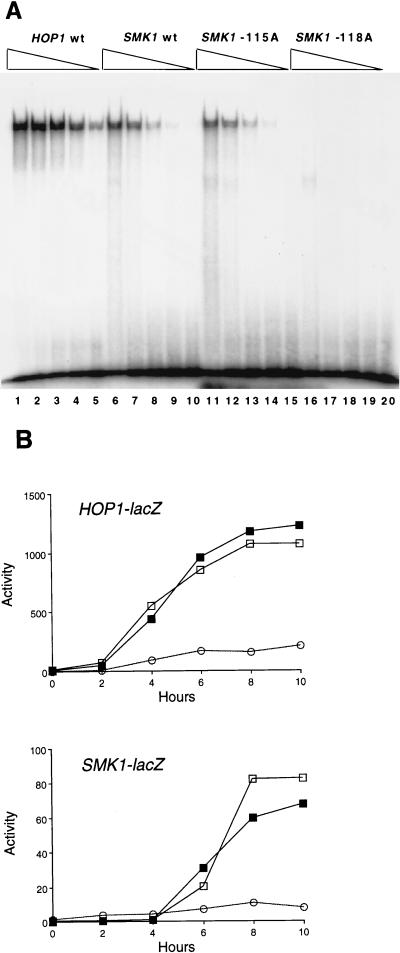
FIG. 2.
Comparison of UASS and UASH sites. (A) EMSA of Abf1p binding. Partially purified Abf1p was serially diluted fivefold and used in binding reactions to radiolabeled 13-bp oligonucleotide duplexes containing the HOP1 Abf1p-binding site (lanes 1 to 5), the wild-type SMK1 Abf1p-binding site (lanes 6 to 10), and the mutant SMK1 Abf1p sites containing the −115A (lanes 11 to 15) and −118A (lanes 16 to 20) substitutions. (B) Analysis of HOP1 and SMK1 promoters containing heterologous Abf1p-binding sites. The HOP1 promoter (upper panel) lacking its Abf1p-binding element (open circles), reconstituted with the HOP1 binding site (open squares), or reconstituted with the SMK1 Abf1 site (closed squares) was tested by using the HOP1-lacZ plasmid pAV130, pCC83, or pJX33, respectively. The SMK1 promoter (lower panel) lacking a functional Abf1p-binding site (−118A mutant; open circles), containing its normal Abf1p-binding site (open squares), or reconstituted with the Abf1p-binding site from HOP1 (closed squares) was tested for expression of β-galactosidase by using the SMK1-lacZ plasmid pMDP126-118A, pMDP126, or pMDP113, respectively, in yeast strain LNY150 as described in Materials and Methods. The experiment was performed independently three times with similar results.