Abstract
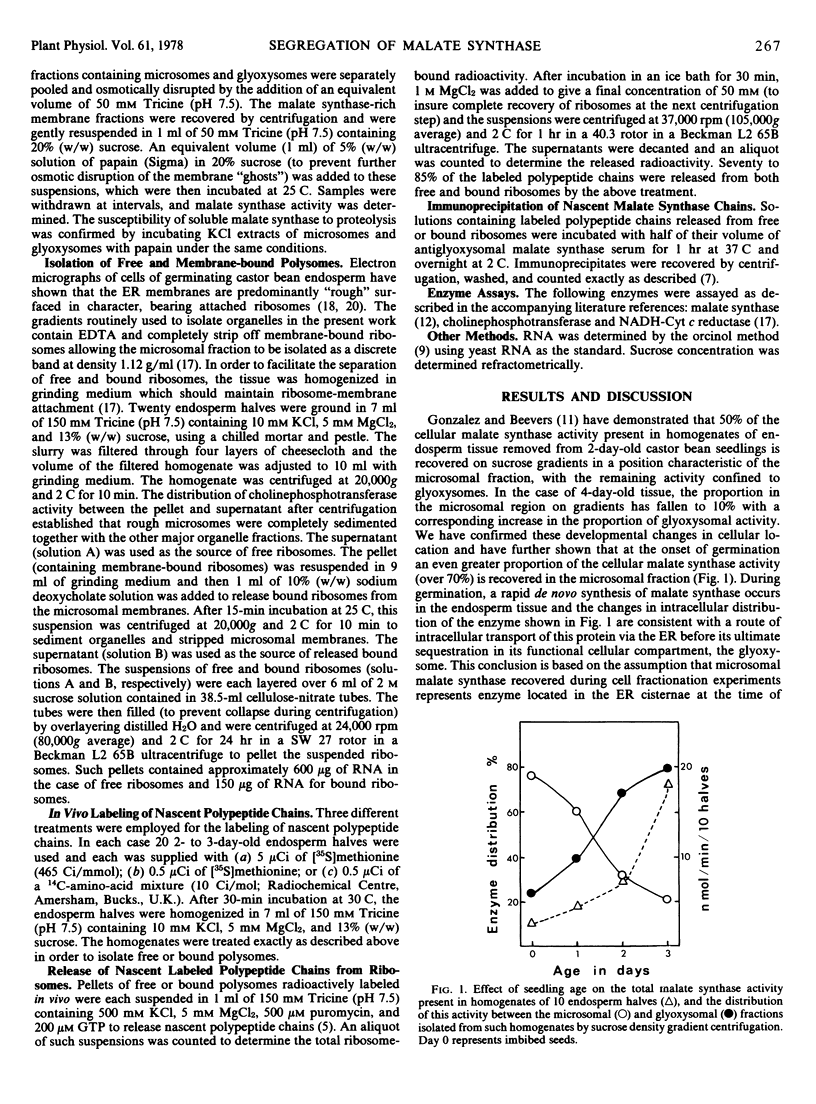
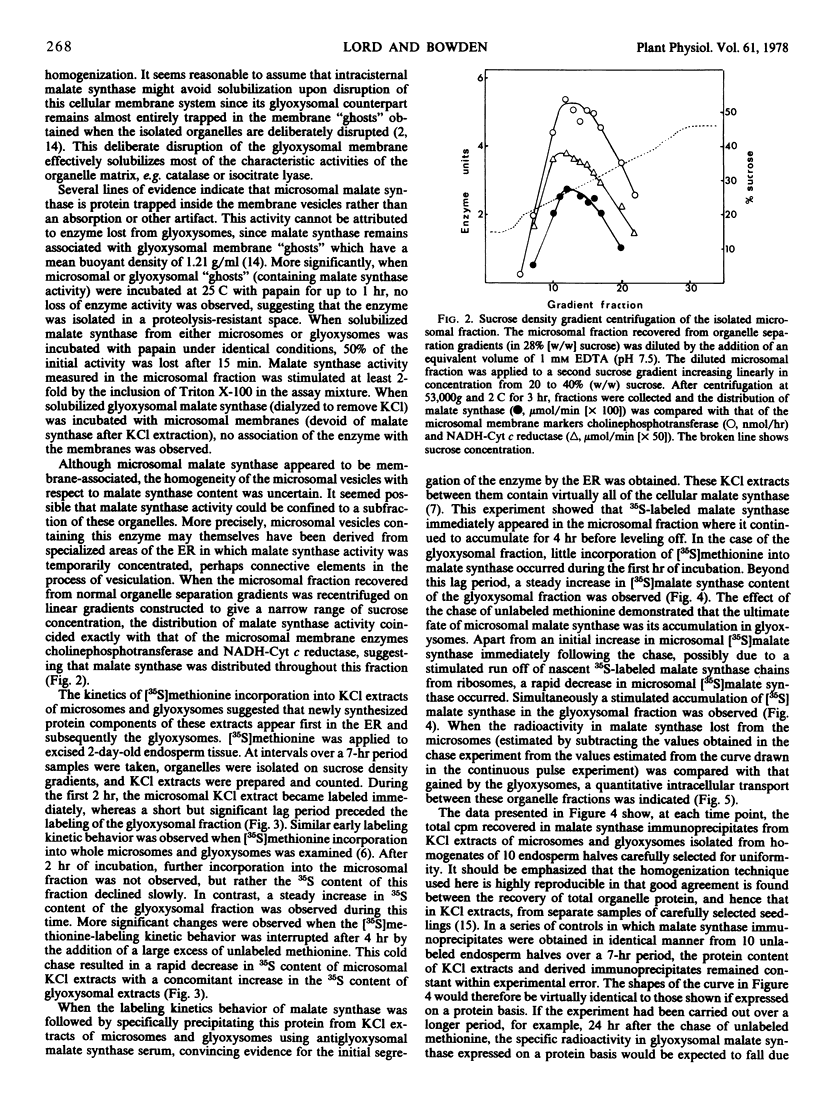
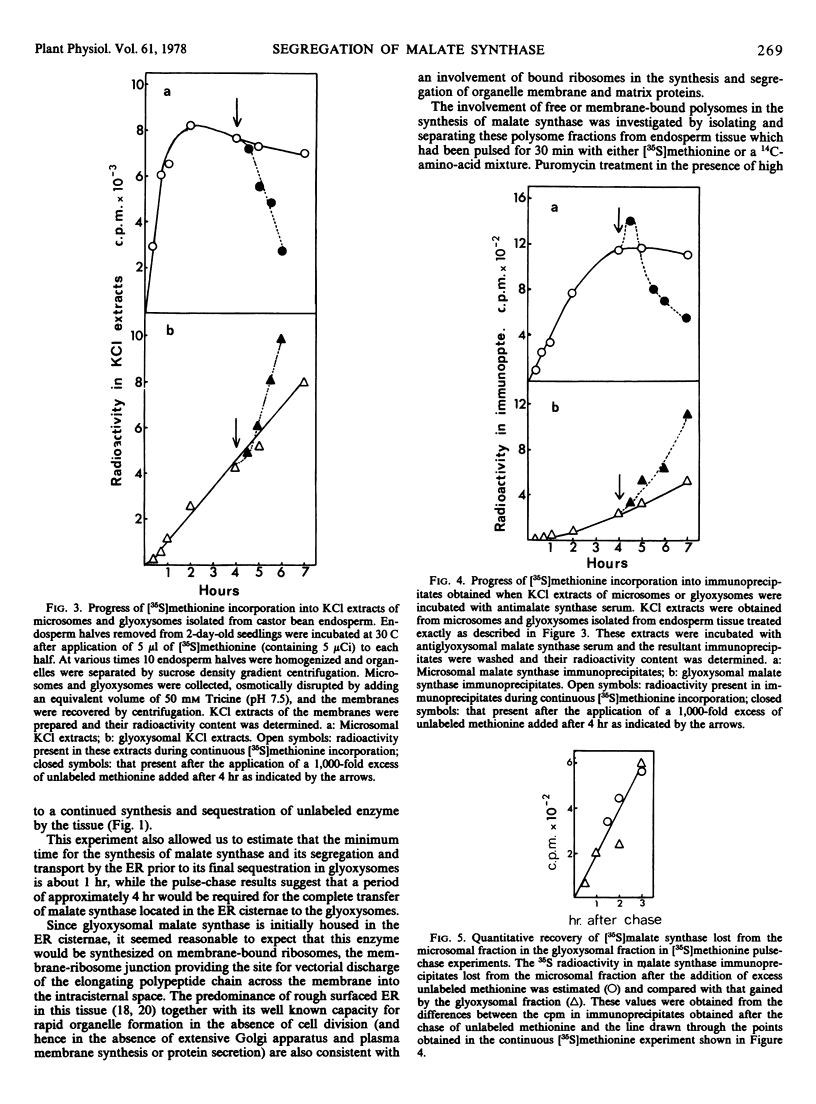
At the onset of castor bean (Ricinus communis) germination, 76% of the cellular malate synthase activity of the endosperm tissue was located in the microsomal fraction, with the remainder in the glyoxysomal fraction. During later developmental stages, when rapid malate synthase synthesis was occurring, an increasing proportion of the enzyme was recovered in glyoxysomes. The kinetics of [35S]methionine incorporation into microsomal and glyoxysomal malate synthase in 2-day-old endosperm tissue was followed by employing antiserum raised against glyoxysomal malate synthase to precipitate specifically the enzyme from KCl extracts of these organelle fractions. This experiment showed that microsomal malate synthase was labeled before the glyoxysomal enzyme. When such kinetic experiments were interrupted by the addition of an excess of unlabeled methionine, 35S-labeled malate synthase was rapidly lost from the microsomal fraction and was quantitatively recovered in the glyoxysomal fraction.
Free cytoplasmic ribosomes were separated from bound ribosomes (rough microsomes) using endosperm tissue labeled with [35S]methionine or 14C-amino-acids. Nascent polypeptide chains were released from polysome fractions using a puromycin-high salt treatment, and radioactive malate synthase was shown to be exclusively associated with bound polysomes.
Together these data establish that malate synthase is synthesized on bound ribosomes and vectorially discharged into the endoplasmic reticulum cisternae prior to its ultimate sequestration in glyoxysomes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Bieglmayer C., Graf J., Ruis H. Membranes of glyoxysomes from castor-bean endosperm. Enzymes bound to purified-membrane preparations. Eur J Biochem. 1973 Sep 3;37(3):553–562. doi: 10.1111/j.1432-1033.1973.tb03018.x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Purification and comparative properties of microsomal and glyoxysomal malate synthase from castor bean endosperm. Plant Physiol. 1978 Feb;61(2):259–265. doi: 10.1104/pp.61.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. The cellular origin of glyoxysomal proteins in germinating castor-bean endosperm. Biochem J. 1976 Feb 15;154(2):501–506. doi: 10.1042/bj1540501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., de Duve C. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J Cell Biol. 1973 Nov;59(2 Pt 1):507–524. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer H. H., Totten C. Studies on seeds: v. Microbodies, glyoxysomes, and ricinosomes of castor bean endosperm. Plant Physiol. 1970 Dec;46(6):794–799. doi: 10.1104/pp.46.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Grab D. J., Irukulla R. The intracellular pathway of newly formed rat liver catalase. Arch Biochem Biophys. 1972 Oct;152(2):496–501. doi: 10.1016/0003-9861(72)90244-5. [DOI] [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]


