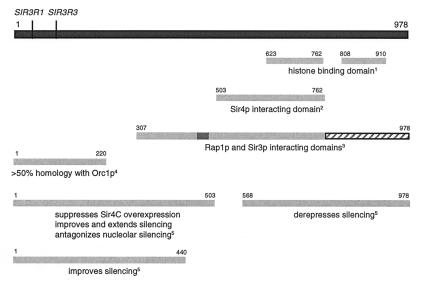
FIG. 2.
Functional domains of Sir3p. A schematic representation of full-length Sir3p and the functional domains revealed by genetic, two-hybrid, and biochemical studies is shown. Notes: 1, reference 15; 2, two-hybrid data indicate that the Sir4p binding domain is 3′ of aa 494 (6), and unpublished pull-down data indicate that there is only one site of interaction, not two as previously suggested (12a, 43), 3, reference 29 (as indicated by the shaded box, the domain necessary and sufficient for Rap1p interaction has been narrowed down to aa 455 to 481 of Sir3p, and the Sir3p homodimerization domain has been defined from aa 762 to the end of the protein [38a]); 4, reference 2; 5, this study. The two mutations isolated as suppressors of histone H4 mutants are labeled SIR3R1 and SIR3R3 (18). See the text for more details.