Abstract
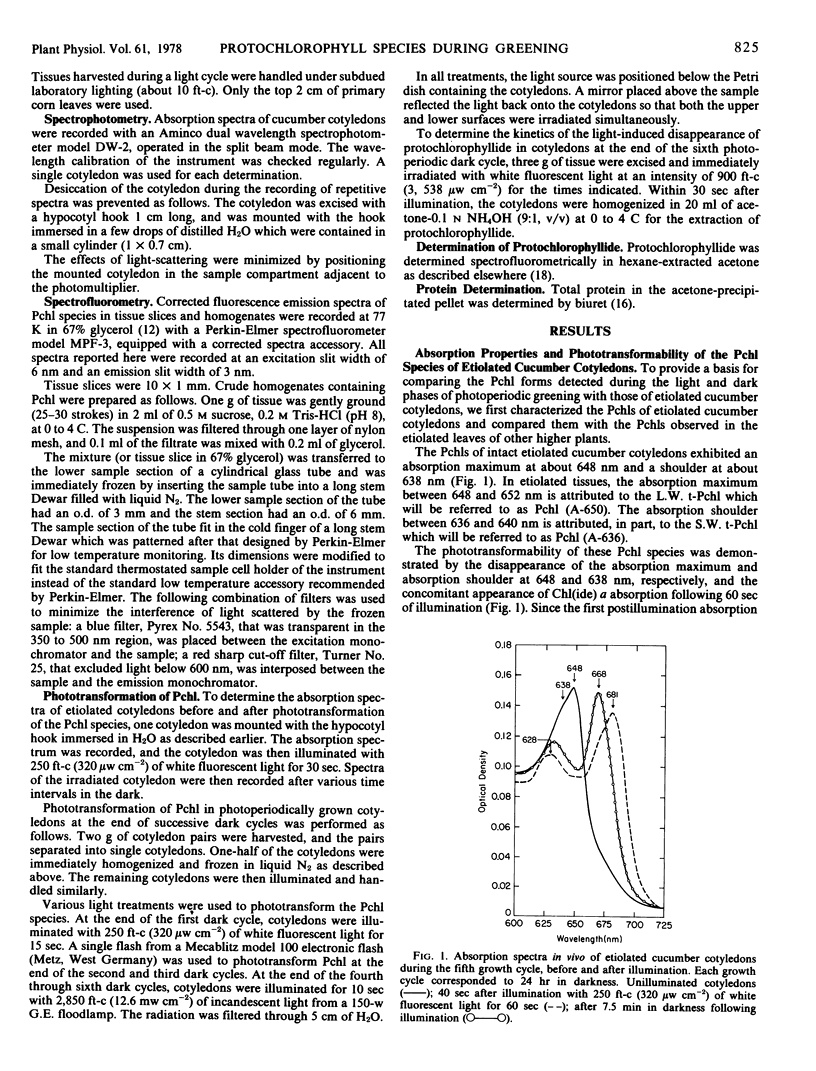
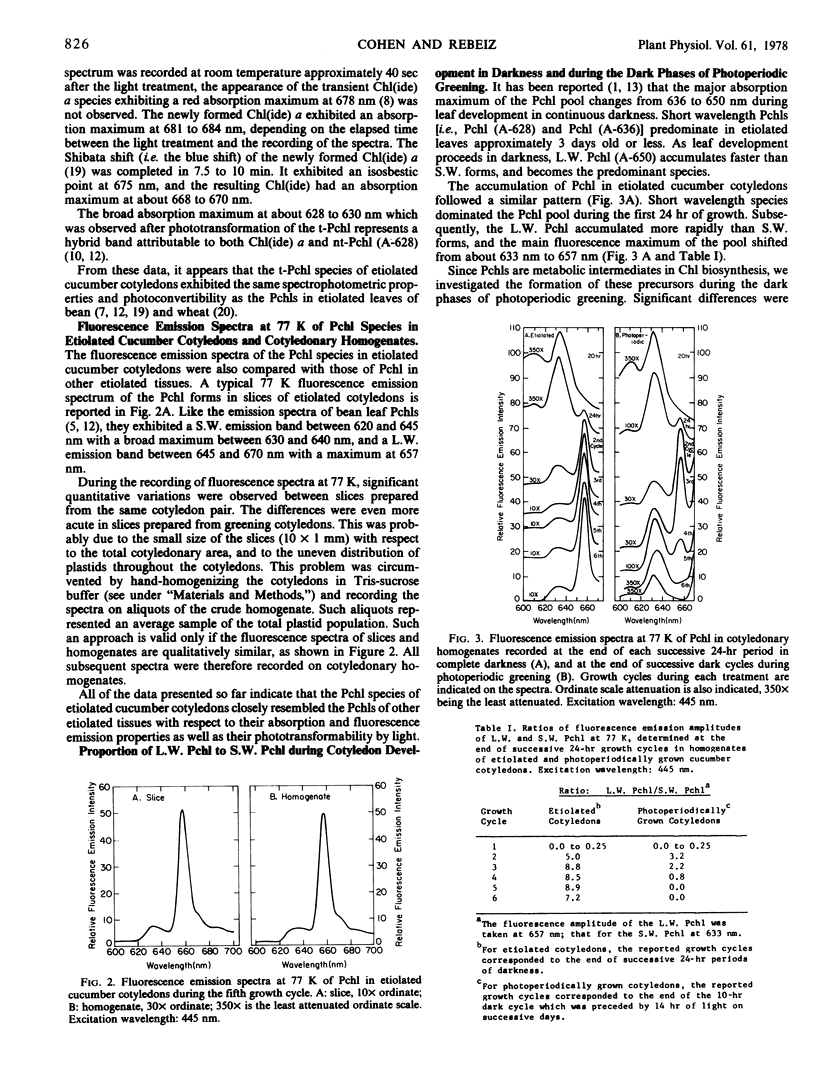
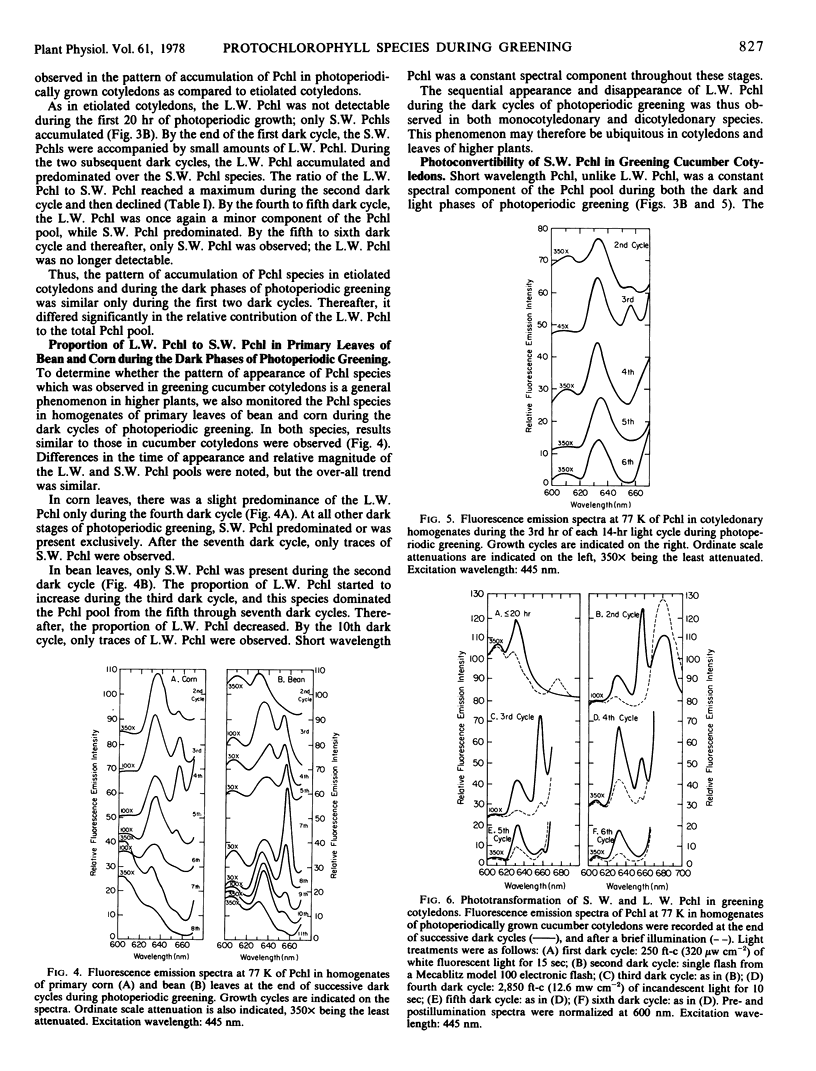
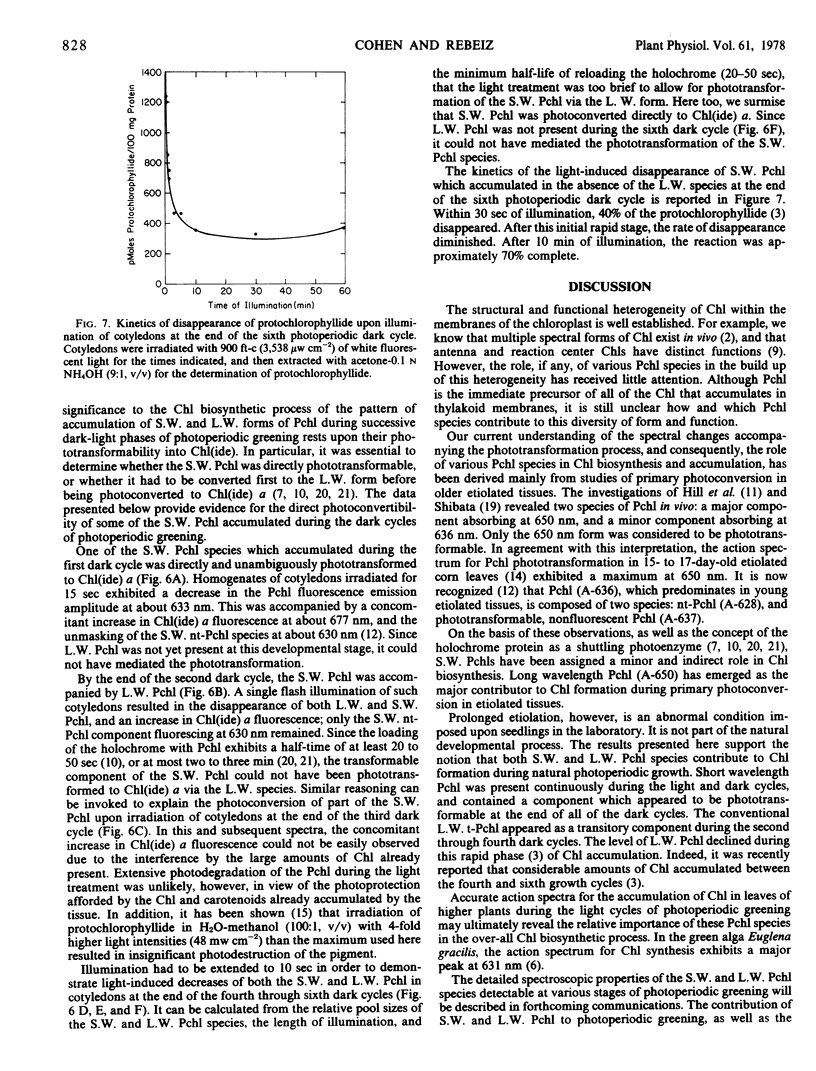
The contribution of short and long wavelength membrane-bound fluorescing protochlorophyll species to the over-all process of chlorophyll formation was assessed during photoperiodic growth. Protochlorophyll forms were monitored spectrofluorometrically at 77 K during the first six light and dark cycles in homogenates of cucumber (Cucumis sativus L.) cotyledons grown under a 14-hour light/10-hour dark photoperiodic regime, and in cotyledons developing in complete darkness. In the etiolated tissue, short wavelength protochlorophyll having a broad emission maximum between 630 and 640 nm appeared within 24 hours after sowing. Subsequently, the long wavelength species fluorescing at 657 nm appeared, and accumulated rapidly. This resulted in the preponderance of the long wavelength species which characterizes the protochlorophyll profile of etiolated tissues. The forms of protochlorophyll present in etiolated cucumber cotyledons resembled those in etiolated bean leaves in their absorption, fluorescence, and phototransformability. A different pattern of protochlorophyll accumulation was observed during the dark cycles of photoperiodic greening. The short wavelength species appeared within 24 hours after sowing. Subsequently, the long wavelength form accumulated and disappeared. The long wavelength to short wavelength protochlorophyll emission intensity ratio reached a maximum (~3:1) during the second dark cycle, then declined during subsequent dark cycles. Short wavelength species were continuously present in the light and dark. Primary corn and bean leaves exhibited a similar pattern of protochlorophyll accumulation. In cucumber cotyledons, both the short and long wavelengths species appeared to be directly phototransformable at all stages of photoperiodic development. It thus appears that whereas the long wavelength protochlorophyll species is the major chlorophyll precursor during primary photoconversion in older etiolated tissues, both long wavelength and short wavelength species seem to contribute to chlorophyll formation during greening under natural photoperiodic conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen C. E., Bazzaz M. B., Fullett S. H., Rebeiz C. A. Chloroplast Biogenesis: XX. Accumulation of Porphyrin and Phorbin Pigments in Cucumber Cotyledons during Photoperiodic Greening. Plant Physiol. 1977 Nov;60(5):743–746. doi: 10.1104/pp.60.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J. M., Dorsky D., Schiff J. A. Events Surrounding the Early Development of Euglena Chloroplasts: VI. Action Spectra for the Formation of Chlorophyll, Lag Elimination in Chlorophyll Synthesis, and Appearance of TPN-dependent Triose Phosphate Dehydrogenase and Alkaline DNase Activities. Plant Physiol. 1975 Aug;56(2):318–323. doi: 10.1104/pp.56.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M. L. The Conversion of Photoinactive Protochlorophyllide(633) to Phototransformable Protochlorophyllide(650) in Etiolated Bean Leaves Treated with delta-Aminolevulinic Acid. Plant Physiol. 1973 Dec;52(6):590–594. doi: 10.1104/pp.52.6.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Granick S., Mauzerall D. A rapid spectral change in etiolated red kidney bean leaves following phototransformation of protochlorophyllide. Biochem Biophys Res Commun. 1968 Jul 26;32(2):295–300. doi: 10.1016/0006-291x(68)90384-7. [DOI] [PubMed] [Google Scholar]
- Granick S., Gassman M. Rapid regeneration of protochlorophyllide(650). Plant Physiol. 1970 Feb;45(2):201–205. doi: 10.1104/pp.45.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSKI V. M., FRENCH C. S., SMITH J. H. C. The action spectrum for the transformation of protochlorophyll to chlorophyll a in normal and albino corn seedlings. Arch Biochem Biophys. 1951 Mar;31(1):1–17. doi: 10.1016/0003-9861(51)90178-6. [DOI] [PubMed] [Google Scholar]
- Kahn A., Boardman N. K., Thorne S. W. Energy transfer between protochlorophyllide molecules: evidence for multiple chromophores in the photoactive protochlorophyllide-protein complex vivo and in vitro. J Mol Biol. 1970 Feb 28;48(1):85–101. doi: 10.1016/0022-2836(70)90220-2. [DOI] [PubMed] [Google Scholar]
- Klein S., Schiff J. A. The Correlated Appearance of Prolamellar Bodies, Protochlorophyll(ide) Species, and the Shibata Shift during Development of Bean Etioplasts in the Dark. Plant Physiol. 1972 Apr;49(4):619–626. doi: 10.1104/pp.49.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REBEIZ C. A., CASTELFRANCO P., ENGELBRECHT A. H. FRACTIONATION AND PROPERTIES OF AN EXTRA-MITOCHONDRIAL ENZYME SYSTEM FROM PEANUTS CATALYZING THE BETA-OXIDATION OF PALMITIC ACID. Plant Physiol. 1965 Mar;40:281–286. doi: 10.1104/pp.40.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Mattheis J. R., Smith B. B., Rebeiz C. C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys. 1975 Dec;171(2):549–567. doi: 10.1016/0003-9861(75)90065-x. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. A., Mattheis J. R., Smith B. B., Rebeiz C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of Mg-protoprophyrin IX monoester and longer wavelength metalloporphyrins by greening cotyledons. Arch Biochem Biophys. 1975 Feb;166(2):446–465. doi: 10.1016/0003-9861(75)90408-7. [DOI] [PubMed] [Google Scholar]
- Süzer S., Sauer K. The sites of photoconversion of protochlorophyllide to chlorophyllide in barley seedlings. Plant Physiol. 1971 Jul;48(1):60–63. doi: 10.1104/pp.48.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]


