Abstract
Liver diseases are a significant global health burden and are among the most common diseases. Ginssennoside Rg3 (Rg3), which is one of the most abundant ginsenosides, has been found to have significant preventive and therapeutic effects against various types of diseases with minimal side effects. Numerous studies have demonstrated the significant preventive and therapeutic effects of Rg3 on various liver diseases such as viral hepatitis, acute liver injury, nonalcoholic liver diseases (NAFLD), liver fibrosis and hepatocellular carcinoma (HCC). The underlying molecular mechanism behind these effects is attributed to apoptosis, autophagy, antioxidant, anti-inflammatory activities, and the regulation of multiple signaling pathways. This review provides a comprehensive description of the potential molecular mechanisms of Rg3 in the development of liver diseases. The article focuses on the regulation of apoptosis, oxidative stress, autophagy, inflammation, and other related factors. Additionally, the review discusses combination therapy and liver targeting strategy, which can accelerate the translation of Rg3 from bench to bedside. Overall, this article serves as a valuable reference for researchers and clinicians alike.
Keywords: Liver diseases, Ginsenoside Rg3, Molecular mechanisms, Combination therapy, Liver targeting strategy
Graphical abstract
1. Introduction
Liver diseases are a significant global health challenge, responsible for approximately 2 million deaths annually worldwide. The high prevalence of liver diseases poses serious public health concerns, given their poor long-term clinical outcomes, including premature deaths resulting from liver decompensation, cirrhosis, and hepatocellular carcinoma (HCC) [1]. A significant portion of the global population is at risk of various liver diseases such as chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, non-alcoholic fatty liver (NAFLD), autoimmune liver disease, and drug-induced liver disease [2]. Currently, drug therapy is the primary clinical treatment for liver diseases. While surgical intervention may be appropriate for early-stage HCC, it has proven to be ineffective with a high recurrence rate. In cases of end-stage liver disease, liver transplantation is the most effective treatment option. However, the limited availability of donor livers and the lifelong medication required post-transplantation can be prohibitive factors in its application [3]. Therefore, it is crucial to find more effective drugs for the therapy of liver diseases.
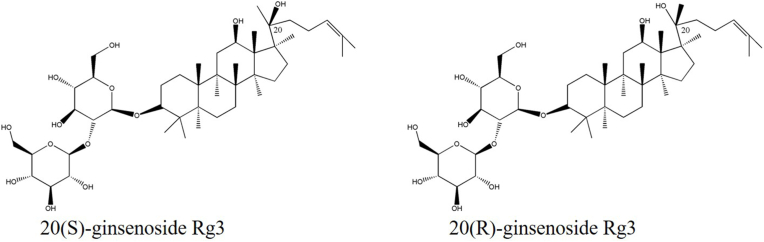
Compared with synthetic drugs, traditional Chinese medicine, as an alternative clinical treatment method, has attracted much attention for its mild and far-reaching curative effect and less side effects. Recent studies have increasingly demonstrated the efficacy of natural active ingredients, including dihydromyricetin, betaine, and kaempferol, in the treatment of liver diseases. These ingredients have been found to possess reliable pharmacological effects, while exhibiting low toxicity and conferring numerous benefits [[4], [5], [6]]. The root of the Panax ginseng Meyer plant, commonly referred to as ginseng, has been traditionally used as a medicine in Asian countries, primarily for its energy-boosting properties [7]. Ginsenoside saponins are the major bioactive components responsible for ginseng's pharmacological effects, with over 100 types of ginsenosides identified and isolated from ginseng [8]. Among these ginsenosides, ginsenoside Rg3 (Rg3) has been found to have significant physiological activity. Rg3 (PubChem CID: 9918693), the chemical name is 12β,20dihydroxydamar-24-en-3β-yl2-o-β-D-glucopyranosyl-β-D-glucopyranosyl-β-D-glucopyranoside, corresponding to its asymmetric carbon atom C20, Rg3 exists in two forms of R type and S type (Fig. 1) [9,10]. Numerous preclinical trials have also shown that Rg3 has a variety of biological activities, including but not limited to antioxidative [11], anti-inflammatory [12] and antitumor [13]. Rg3 has been found to have various hepatoprotective effects such as inhibition of viral hepatitis, improvement of acute liver injury, relief of NAFLD and liver fibrosis, and reduction in the occurrence of HCC. However, the mechanisms responsible for the therapeutic benefits of Rg3 in liver diseases are yet to be established.
Fig. 1.
The Chemical structures of 20(S)-Rg3 and 20(R)-Rg3.
This review systematically describes the molecular mechanisms of Rg3's hepatoprotective effects on liver diseases, as well as the underlying mechanisms of Rg3's new formulations and carriers. The aim is to promote the development of novel therapeutic methods for liver diseases.
2. The role of Rg3 in liver diseases
Rg3 has been found to have various biological activities that have led to its use in preventing and treating different systemic diseases in vivo. Recent studies have shown that Rg3 plays a crucial role in preventing viral hepatitis, acute liver injury, and liver fibrosis. It has also been found to alleviate NAFLD and HCC (Table 1).
Table 1.
Pharmacological Activities of Rg3 in the Treatment of Liver Diseases
| Disease | Model | Types | Dosage | Effects and related mechanisms | References |
|---|---|---|---|---|---|
| Hepatitis B virus | HepG2.2.15 | In vitro | 100 μM | Downregulation: JNK, AP-1, HBsAg and HBeAg Upregulation: TRAF6, TAK1, IL-8 and TNF-α |
[20] |
| Hepatitis C virus | Huh7 Huh7.5.1 | In vitro | 100 μM | Downregulation: CDK1 and Drp1 Upregulation: p21 |
[24] |
| Liver injury | HepG2 t-BHP-induced male ICR Mice |
In vitro In vivo |
1-10 μM 25, 50 mg/kg |
Downregulation: ALT and AST | [42] |
| Liver injury | LPS-induced liver injury in Male Wistar rats | In vivo | 5, 10 mg/kg | Downregulation: GOT, GPT, NF-kB, COX-2 and iNOS Upregulation: HO-1 |
[43] |
| Liver injury | Cisplatin-induced liver injury in Male BALB/c mice | In vivo | 5, 10 mg/kg | Downregulation: BUN, ALT, AST, NO, MDA, ROS and 3-NT Upregulation: GSH-Px, SOD and CAT |
[40] |
| Liver injury | EtOH induced TIB-73 | In vitro | 1-30 μM | Downregulation: LDH, AST, ROS, ERK and JNK | [41] |
| Liver injury | NAPQI induced H4IIE | In vitro | 1-10μg/mL | Downregulation: ALT, AST, Mrp2 and Mrp4 Upregulation: GSH, Nrf2, Mrp1, Mrp3, GCLC and GCLM |
[29] |
| Liver injury | Human primary hepatocytes CLP induced-C57BL/6 mice |
In vitro In vivo |
6.25, 12.5, 25μM 10, 20 mg/kg |
Downregulation: ROS, GST Upregulation: OCR, GSH, OPA1, Complex I, and Complex II, PGC1-α, NRF-1, Tfam-1, LC3B II/LC3B I, Beclin-1, AMPK and ACC |
[34] |
| Liver injury | APAP-induced liver injury in Male ICR mice | In vivo | 10, 20 mg/kg | Downregulation: ALT, AST, MDA, 4-HNE, CYP2E1, Bax, TNF-α, IL-1β, IKKα, IKKβ, IκBα and NF-κB Upregulation: GSH, PI3K, AKT and Bcl-2 |
[30] |
| Liver injury | D-Galactose Male ICR mice |
In vivo | 10, 20 mg/kg | Downregulation: ALT, BUN, AGEs, MDA, 4-HNE, CYP2E1, Bax, p53 and cleaved-caspase3 Upregulation: CAT, SOD, PI3K, AKT and Bcl-2 |
[37] |
| Liver injury | Human primary hepatocytes CLP induced-C57BL/6 mice |
In vitro In vivo |
25 μM 20 mg/kg |
Upregulation: OPA, Complex I, and Complex II, PGC1-α, NRF-1, Tfam-1, LC3B II, LC3B I, Beclin-1, TUG1, SIRT1, AMPK and ACC | [35] |
| NAFLD | HepG2 | In vitro | 1-100 μM | Downregulation: TC, TG, SREBP-2 and HMGCR Upregulation: AMPK |
[48] |
| NAFLD | 3T3-L1 HFD-induced C57BL/6 male mice |
In vitro In vivo |
5-50 μM 1mg/kg |
Downregulation: TC, TG, ALT, AST, STAT5, PPARγ, TNF-α, IL-1β, Fabp4, Scd1 and CPT-1α Upregulation: p-AKT, IL-6 and IL-10, |
[49] |
| NAFLD | HFD-induced C57BL/6 mouse, db/db mice | In vivo | - | Downregulation: pro-inflammatory cytokine secretion | [53] |
| NAFLD | HFD-induced C57BL/6 mouse | In vivo | - | Downregulation: TC, LDL, TG and AST | [50] |
| NAFLD | Primary hepatocytes Raw264.7 cells |
In vitro | 5 μM | Downregulation: mTORC1, Ccl2, Ccl5, IL-1β, IL-6, iNos and TNF-α Upregulation: Hmgcs2, CD163 and IL-10 |
[54] |
| NASH | Macrophage-induced THP-1 Male C57BL/6J mice |
In vitro In vivo |
0.001–1 μg/mL 15, 30 mg/kg |
Downregulation: F4/80 and p–NF–κB | [55] |
| Liver fibrosis | LPS-induced HSC-T6 TAA-induced liver injury in Male ICR mice |
In vitro In vivo |
0-16 μM 5, 10 mg/kg |
Downregulation: AST, ALT, CAT, MDA, TGF-β1 and α-SMA, LC3b/LC3a, p62, ATG5 and ATG7 Upregulation: SOD, GSH, PI3K, AKT and mTOR |
[63] |
| HCC | SMMC-7721 | In vitro | 15 μg/ml | Downregulation: PCNA and cyclin D1 | [74] |
| HCC | Hep3B | In vitro | 1-30 μM | Downregulation: Bcl-2 Upregulation: LDH, ROS, Bax, caspase-3 and cytosolic cytochrome c |
[68] |
| HCC | Hep1-6 HepG2 C57BL/6 mice |
In vitro In vivo |
0-200 μg/ml 3.0 mg/kg |
Downregulation: Bcl-2, Bcl-XL and Cytochrome c in the mitochondrial fraction Upregulation: Bax and Cytochrome c in the cytosolic fraction. |
[69] |
| HCC | SMMC-7721 HepG2 |
In vitro | 25-100 μg/ml | Downregulation: Bcl-2 Upregulation: caspase-3 and Bax |
[67] |
| HCC | Primary rat hepatocytes HepG2 |
In vitro | 10-100 μM | Downregulation: OPA-1, Bcl-2 Upregulation: UCP-2, cleaved PARP, Fas and LC3 II |
[70] |
| HCC | KM mice | In vivo | 3.0 mg/kg | Upregulation: IL-2 and IFN-γ | [71] |
| HCC | HepG2 | In vitro | 7.81-500 μg/ml | Downregulation: Bcl-2, VEGF, DNMT3a and DNMT3b Upregulation: P53 |
[72] |
| HCC | Bel-7402 HCCLM3 Male BALB/c nude mice |
In vitro In vivo |
0-100 μM 10 mg/kg |
Downregulation: NHE1, EGF, EGFR, ERK1/2, HIF-1α and Ki67 Upregulation: cleaved-caspase-3 |
[73] |
| HCC | Female C57BL/6 mice | In vivo | 10 mg/kg | Downregulation: Angiogenesis quantification | [80] |
| HCC | HepG2 MHCC-97L BALB/c nude mice |
In vitro In vivo |
1.25-5 μg/ml 2.5-10 mg/kg |
Upregulation: ARHGAP9 | [79] |
| HCC | SMMC-7721 SK-Hep-1 |
In vitro | 1- 16 μg/ml | Downregulation: lncRNA-HOTAIR, MMP2, MMP9, PI3K and AKT | [77] |
2.1. Rg3 in viral hepatitis
Viral hepatitis is a significant worldwide health concern that affects millions of individuals. Despite recent medical advancements, it continues to be linked with high levels of illness and death [14]. Viral hepatitis is primarily caused by five liver-specific viruses, namely hepatitis A virus (HAV), hepatitis B virus, hepatitis C virus, hepatitis D virus (HDV), and hepatitis E virus (HEV). Among these, hepatitis B and C are responsible for 96% of viral hepatitis mortality [14,15].
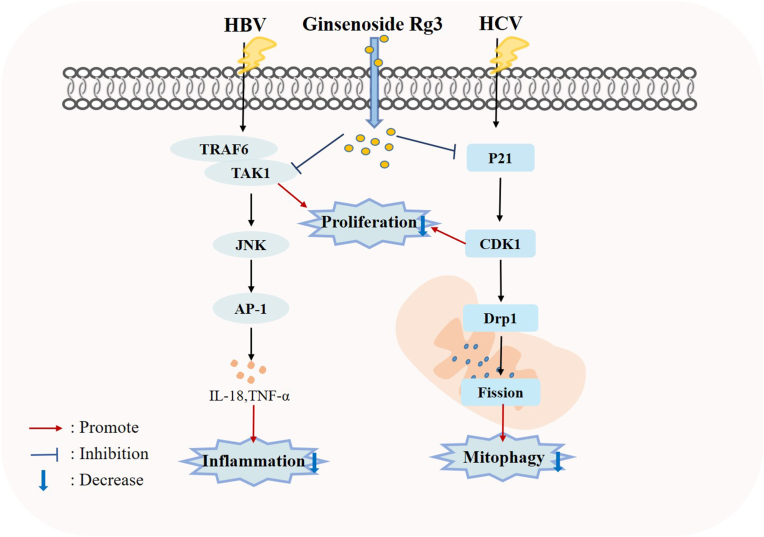
HBV infection is a severe public health concern that poses a significant risk to human health. Currently, there are over 250 million individuals worldwide who have contracted HBV, resulting in nearly one million deaths each year [16]. HBV is a virus that targets the liver and is composed of a partially double-stranded circular DNA genome. This genome is often integrated into hepatocellular DNA, causing both acute and chronic liver damage [17]. Chronic hepatitis B is primarily caused by the immune system's response to the virus as the virus itself does not harm liver cells. This results in a prolonged phase of immune tolerance, where individuals are persistently infected with high levels of the virus and are HBeAg-positive. Chronic infection is strongly linked to the development of HCC in humans [18,19]. According to report, Rg3 has shown potential as an anti-hepatitis B agent by inhibiting HBsAg, HBeAg, viral particle secretion, and viral replication in a dose and time-dependent manner. The anti-HBV activity of Rg3 is associated with downregulation of TNF receptor-associated factor 6 (TRAF6) and transforming growth factor-β-activating kinase 1 (TAK1) through stimulation of TRAF6 degradation. Rg3 also inhibits HBsAg secretion and interleukin 8 (IL-8)/tumor necrosis factor-α (TNF-α) expression, specifically downregulates the c-Jun/JunB complex and inhibits the AP-1 promoter activity [20].
HCV is a significant contributor to the development of hepatocellular carcinoma and end-stage liver disease, which frequently necessitates liver transplantation. The World Health Organization estimates that approximately 71 million people worldwide are affected by HCV, with at least 400,000 deaths annually attributed to the virus [21,22]. Unlike other viral infections, HCV infection typically results from long-term exposure to the virus, leading to chronic infection in most patients [21]. HCV infections can cause damage to mitochondria, leading to oxidative stress, disruption of mitochondrial membrane potential, and dysfunction. This can ultimately result in apoptosis [23]. Rg3 was found to be an effective inhibitor of HCV propagation. This is due to its ability to restore abnormal mitochondrial fission and subsequent mitophagy that is induced by HCV. This is achieved by inhibiting the activation of the cyclin-dependent kinase 1 (CDK1)-dynamin-related protein 1 (Drp1) pathway. The activity of CDK1 is modulated by cytosolic p21, which is suppressed by HCV core protein. However, Rg3 was able to restore the expression level of p21, thus inhibiting the CDK1-Drp1 pathway and preventing abnormal mitochondrial fission and mitophagy [24]. According to this study, Rg3 has been found to work in synergy with nucleotide inhibitors, such as sofosbuvir, making it a valuable candidate for treating HCV patients. Rg3 can be used as a monotherapy or in combination with sofosbuvir [24]. Additionally, ongoing research is being conducted to explore the potential application of Rg3 in treating other viral infections [25].
Collectively, these results indicate that Rg3 may be useful in therapy for viral hepatitis, with the underlying mechanisms such as regulation of TRAF6/TAK1/JNK/AP-1 pathway and inhibiting activation of the CDK1-Drp1 signaling pathway (Fig. 2). Notably, due to the lack of data supporting systematic animal studies, further research and translation are required before these results can be translated into clinical trial.
Fig. 2.
Cellular and molecular mechanisms of Rg3 in the prevention of viral hepatitis.
2.2. Rg3 in liver injury
Drug metabolism in the liver is a complex process, and the accumulation of drugs or their byproducts can lead to severe liver damage and dysfunction [26]. Acetaminophen (APAP), a traditional nonsteroidal antipyretic analgesic, is often associated with drug-induced liver injury and even acute liver failure. N-acetyl-p-benzoquinone imine (NAPQI) is a toxic metabolite, and high doses of APAP can cause liver necrosis [27]. In a recent study, Gum et al found that Rg3 can modulate APAP metabolism by inhibiting cytochrome P450 2E1 (CYP2E1) and inducing glutathione S-transferase A2. This resulted in the amelioration of APAP-related hepatocyte necrosis, which was found to be associated with an increase in nuclear factor-erythroid 2-related factor 2 (Nrf2) [28]. Simultaneously, they also found the ameliorative effects of Rg3 on NAPQI-induced hepatotoxicity in a rat model [29]. In another study, Zhou et al found that Rg3 treatment effectively reduced the depletion of GSH, production of malondialdehyde (MDA), and overexpression of CYP2E1 and 4-hydroxynonenal (4-HNE) caused by injection of APAP. They also discovered that Rg3 significantly alleviated APAP-induced apoptosis, inflammatory infiltration, and necrosis in liver through activating the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT) pathway [30].
Sepsis has a high mortality rate and is a major threat to human health. Strategies focusing on reducing liver injury and restoring liver function should be implemented to reduce morbidity and mortality in patients with sepsis. Clinical severity and organ dysfunction are closely related to sepsis-induced mitochondrial impairment [31,32]. Mitophagy, the process by which cells remove damaged mitochondria, protects them from sepsis-induced organ dysfunction [33]. Previous study has indicated that Rg3 has the potential to promote oxygen consumption rate (OCR), reduce reactive oxygen species (ROS), and maintain GSH pools and its conjugating activity in vitro models. Furthermore, Rg3 treatment has been found to suppress mitochondrial dysfunction through increasing the protein expression levels of mitochondrial biogenesis-related transcription factors. In sepsis models, Rg3 has been shown to enhance the production of autophagy-related proteins and activate silencing information regulator 1 (SIRT1)-AMP-activated protein kinase (AMPK) signaling pathway. However, in LPS-induced human primary hepatocytes, the protective function of Rg3 on mitochondria decreased when autophagy inhibitors or AMPK inhibitors were used [34]. In another study, it was found that Rg3 increased taurine-upregulated gene 1 (TUG1) expression and reduced miR-200a-3p expression, which stimulated the SIRT1/AMPK pathway. This resulted in enhanced autophagy and improved sepsis-induced liver injury and mitochondrial dysfunction [35].
Excessive consumption of D-galactose, a common reducing sugar, can lead to the accumulation of ROS and stimulate free radical production by forming advanced glycation end products in tissues. This can result in oxidative stress, leading to dysfunction in multiple organs and systems within the body. Additionally, the activity of antioxidant enzymes in various organs may be reduced [36]. The treatment of Rg3 can alleviate D-galactose-induced liver and kidney damage in aged mice by inhibiting oxidative stress and PI3K/AKT-mediated apoptosis [37]. Cisplatin can be considered a double-edged sword. It is widely used in the treatment of various solid tumors such as ovarian cancer, prostate cancer, testicular cancer and colon cancer [38]. At the same time, it is toxic to multiple tissues and can cause nephrotoxicity, hepatotoxicity and cardiotoxicity [39]. In a recent study, Lee et al discovered that supplementing with Rg3 can offer protection to the kidney and liver by preventing the generation of intracellular ROS induced by cisplatin. Additionally, it can reduce the expression of Nrf2-mediated heme oxygenase-1 (HO-1)/NQO-1. This finding suggests that Rg3 supplementation could potentially be used as a preventative measure against tissue damage in the kidney and liver [40]. Park et al also demonstrated that Rg3 has a protective effect against EtOH-induced hepatocytic injury by reducing the levels of ROS and the activation of ERK and JNK signaling pathways [41]. In addition, the treatment of Rg3 potently inhibited t-BHP-induced cytotoxicity in HepG2 cells, and orally administered Rg3 showed a potent hepatoprotective effect in vivo [42]. Moreover, another study showed that the preventive effect of Rg3 against lipopolysaccharide (LPS)-induced acute oxidative damage in the liver [43].
In summary, Rg3 exhibits potential hepatoprotection effects against liver injury through multiple mechanisms associated with PI3K/AKT pathways, Nrf2 pathways, SIRT1/AMPK signaling and ERK/JNK pathways (Fig. 3).
Fig. 3.
Mechanisms for Rg3 ameliorating liver injury.
2.3. Rg3 in NAFLD
Non-alcoholic fatty liver disease (NAFLD) encompasses a range of conditions, from simple steatosis to nonalcoholic steatohepatitis (NASH), cirrhosis, and even hepatocellular carcinoma. The global prevalence of NAFLD is currently around 25% [44,45], making it the most common cause of chronic liver disease and the leading reason for liver failure requiring transplantation in Western countries [46,47]. The development of NAFLD is multifactorial and involves various factors including insulin resistance, lipid accumulation, oxidative stress, mitophagy, cytokines, and inflammatory mediators [44]. Unfortunately, there are no effective targeted therapies to treat NAFLD. Rg3 has a wide range of functions and can play a strong preventive role in the occurrence and development of NAFLD from multiple perspectives.
In HepG2 cells, Rg3 was found to decrease the accumulation of hepatic lipids, increase the activity of AMPK, and inhibit the expression of sterol regulatory element binding protein-2 (SREBP-2) and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR). Additionally, Rg3 reduced levels of hepatic cholesterol (TC) and triglycerides (TG) [48]. Treatment with Rg3 could reduce body weight and enhance insulin sensitivity in the rat liver induced by a high-fat diet (HFD). Furthermore, Rg3 suppressed signal transducer and activator of transcription 5 (STAT5), reduced peroxisome proliferator-activated receptor gamma (PPARγ) target lipogenesis proteins related to lipid accumulation such as fatty acid binding protein 4 (Fabp4), stearoyl-coenzyme A desaturase 1 (Scd1) and carnitine palmitoyltransferase-1α (CPT-1α) [49]. Studies have shown that Rg3 has the potential to regulate the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), which are commonly used as markers of liver damage. Moreover, Rg3 has been found to reduce the incidence of postoperative serum liver failure and hepatic TNF-α levels in mice fed a high-fat diet. Rg3 has also been observed to decrease hepatic lipids, including TG, thereby inhibiting hepatic steatosis by reducing lipid accumulation [50].
Inflammation is strongly linked to acute and chronic liver diseases. The activation of inflammatory signaling pathways, such as nuclear factor kappa B (NF-κB), JNK, and p38 MAPK, can lead to the production of numerous cytokines and inflammatory factors. These factors can collectively contribute to the onset of inflammation, apoptosis, and fibrosis, ultimately promoting the progression of NAFLD [51,52]. In dyslipidemic and db/db mice, the use of Rg3 and probiotics was found to improve symptoms of non-alcoholic fatty liver disease (NAFLD). This was achieved by reducing liver inflammation through the downregulation of cytokines like interleukin 1β (IL-1β) [53]. Furthermore, Treatment with Rg3 significantly reduced the level of mechanistic target of rapamycin complex 1 (mTORC1) and p–NF–κB expression, increased the marker for M2 (CD163 and IL-10), while decreased the marker for M1 such as chemokine C–C motif ligand 2(Ccl2), chemokine C–C motif ligand 5 (Ccl5), interleukin-6 (IL-6), iNos, IL-1β, and TNF-α in the inflammatory response induced by LPS [54,55].
In short, Rg3 exhibits potential for NAFLD therapy and the hepatoprotective mechanisms including regulating lipid metabolism and anti-inflammatory effects (Fig. 4). Future studies will need to demonstrate whether the underlying mechanism is related to other factors, such as apoptosis, mitophagy and oxidative stress.
Fig. 4.
Cellular and molecular mechanisms of Rg3 in the prevention of non-alcoholic liver diseases.
2.4. Rg3 in liver fibrosis
Liver fibrosis is a response that occurs when the liver attempts to heal wounds caused by acute or chronic liver injuries from various factors, including alcohol consumption, non-alcoholic steatohepatitis (NASH), HBV and HCV [56]. If left untreated, 75-80% of these conditions can progress to cirrhosis or even HCC, which can pose a serious threat to human health [57]. Hepatic stellate cells (HSCs) are believed to play a crucial role in the development of liver fibrosis [58,59]. According to reports, activated HSCs migrate to damaged areas in the liver along with leukocyte infiltration. Inflammatory cells are responsible for hepatic inflammation and secrete proinflammatory and profibrotic factors, such as TGF-β1. This factor, in turn, activates HSCs, leading to the formation of an autocrine-positive feedback loop that further aggravates hepatic fibrogenesis [[60], [61], [62]]. Despite great progress in understanding the etiology of liver fibrosis, specific treatments are still lacking. However, in thioacetamide (TAA)-chronic models, Rg3 has been found to alleviate hepatic pathological changes, reverse hepatic fibrosis, decrease deposition of collagen fibers, reduce expression of HSCs activation marker (α-SMA), and reduce secretion of profibrogenic factors (TGF-β1). In addition, Rg3 was found to have a positive effect on rat hepatic stellate cells (HSC-T6) by inhibiting their survival, while not causing any harm to human hepatocytes. Furthermore, the treatment of Rg3 had a dose-dependent effect on autophagy, resulting in decreased expression of p62 and fewer LC3a transformations into LC3b in LPS-induced rat HSC-T6 cells. Additionally, Rg3 was found to enhance the phosphorylation of PI3K and AKT both in vivo and in vitro [63].
In conclusion, Rg3 has a significant anti-fibrotic effect, but its underlying mechanism needs further study.
2.5. Rg3 in hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is a prevalent cancer, accounting for 70-90% of primary liver cancer cases worldwide [64]. Unfortunately, most patients are diagnosed at advanced stages, resulting in limited treatment options and a poor prognosis [65]. The prevailing therapies for HCC include surgical treatment, liver transplantation, and local ablation. However, the overall 5-year survival rate is less than 16% [66].
2.5.1. Single therapy of Rg3
Recent studies have found that Rg3 suppresses the proliferation of human hepatocellular carcinoma cells via impairment of mitochondrial architecture, inhibition of Bcl-2 protein, stimulating the release of mitochondrial cytochrome c, activation of caspase-3 and Bax protein, trigger autophagy, and production of intracellular ROS [[67], [68], [69], [70]]. In addition, Rg3 has the ability to inhibit the growth of H22 hepatomas and increase the concentration of cytokines such as interleukin-2 (IL-2) and interferon-gamma (IFN-γ) in the immune organs and serum of mice with tumors. These effects were observed without any negative impact in both in vitro and in vivo experiments [71]. In a separate study, it was found that Rg3 had a dose-dependent inhibitory effect on the proliferation of HepG2 cells. It also caused a decrease in the methylation of global genomic DNA and upregulated the expression of DNA methyltransferase (DNMT1) while downregulating the expression of DNMT3a and DNMT3b for a promoter region of a specific gene [72]. Rg3 has been found to have the potential to inhibit the proliferation of HCC and induce apoptosis through in vivo and in vitro studies. This is achieved by reducing the expression and activity of Na+/H+ exchanger 1 (NHE1). Additionally, Rg3 can decrease NHE1 expression by integrally inhibiting the epidermal growth factor (EGF)-EGF receptor (EGFR)-extracellular signal-regulated kinase 1/2 (ERK1/2)-hypoxia-inducible factor 1 alpha (HIF-1α) pathway [73]. Moreover, Rg3 may inhibit the proliferation of hepatocellular carcinoma and promote cell apoptosis via downregulating proliferating cell nuclear antigen (PCNA) and cyclin D1 [74].
The metastatic cascade, which is responsible for over 90% of cancer-related deaths, involves the invasion, migration, and entry of tumor cells into the circulation [75]. Studies have shown that high levels of the lncRNA HOX antisense intergenic (HOTAIR) are linked to hepatocarcinogenesis and metastasis. Additionally, overexpression of this lncRNA is a predictor of tumor recurrence in HCC [76]. Pu et al found that the use of Rg3 led to a significant decrease in the expression of lncRNA-HOTAIR and suppressed the invasion and migration of SK-Hep-1 and SMMC-7721 cells. This effect was observed in conjunction with decreased expression of p-PI3K, p-AKT, matrix metalloproteinase-2 (MMP2) and MMP9. The researchers also found that overexpression of lncRNA-HOTAIR reversed the therapeutic effects of Rg3 [77].
Rho GTPase activating protein 9 (ARHGAP9), a member of the Rho GAP family, has been found to suppress the migration and invasion of hepatocellular carcinoma cells [78]. It was reported that Rg3 caused marked inhibition of cell migration and invasion of human liver cancer cells, HepG2 and MHCC-97L, in vitro, and the growth of HepG2 and MHCC-97L tumors in BABL/c nude mice. The mechanism that Rg3 inhibited the metastasis and invasion of liver cancer is related to expression of ARHGAP9 [79]. Tumor angiogenesis is a crucial process for tumor growth and metastasis as it facilitates the formation of new blood vessels that supply oxygen and nutrients. In an orthotopic HCC tumor model, treatment with Rg3 led to the suppression of vascular endothelial growth factor (VEGF) expression and initiated tumor apoptosis. This resulted in a reduction in tumor volume and weakened its ability to produce a vascular network, thereby hindering further tumor growth and distant metastasis [80]. Therefore, Rg3 could potentially reduce HCC metastasis.
As mentioned above, numerous experiments have shown that Rg3 has both preventive and therapeutic effects on various types of tumors. Its mechanism has been extensively researched and found to be related to several signaling pathways such as lncRNA HOTAIR/PI3K/AKT, NHE1/EGF-EGFR-ERK1/2-HIF-1α, IFN-γ/IL-2, ROS/LC3 II, ARHGAP9, and PCNA/cyclin D1 (as shown in Fig. 5). However, despite these findings, the specific mechanism of Rg3 remains unclear and requires further investigation.
Fig. 5.
Mechanisms for Rg3 therapeutic potential in liver cancers.
2.5.2. Combination therapy of Rg3
From an experimental aspect, Rg3 has synergistic functions in combination with other drugs (Table 2). Sorafenib is a multi-kinase inhibitor used in the treatment of hepatocellular carcinoma. While it inhibits the MAPK signaling pathway, it has been found to activate the PI3K/AKT signaling pathway, which in turn cross-talks with the MAPK pathway, ultimately resulting in drug resistance [[81], [82], [83]]. The combination of sorafenib and Rg3 led to an increase in the expression of phosphatase and tensin homolog (PTEN), Bax, and cleaved caspase-3, while decreasing the expression of AKT. This combination also resulted in a decrease in tumor volumes and weight in vivo [84]. In a separate research study, the combination therapy of Rg3 and sorafenib was found to be effective in alleviating the progression of HCC by regulating glycolysis and suppressing the PI3K/AKT pathway [85]. In addition, the combination of Rg3 and artesunate was able to overcome sorafenib resistance in experimental models. The underlying mechanisms were found to be related to the inhibition of signal transducer and activator of transcription 3 (STAT3) signaling as well as modulation of ROS/STAT3 signaling [86].
Table 2.
Combination Therapy of Rg3 in HCC
| Drug or TACE | Model | Types | Dosage | Activity and Mechanisms | References |
|---|---|---|---|---|---|
| Doxorubicin | In vitro In vivo |
SK-Hep1 HepG2 Huh-7 Hep3B Athymic BALB/c nude mice |
Doxorubicin (2.5μM) Rg3 (100 μM) Doxorubicin (1mg/kg) Rg3 (20 mg/kg) |
Sensitized doxorubicin-induced cancer cell death and Inhibited autophagy possibly by blocking lysosomal function via regulating gene expression such as CHOP | [88] |
| Recombinant human TRAIL | In vitro In vivo |
SK-Hep1 HepG2 Hep3B Huh-7 Male nude mice |
TRAIL (25 ng/mL) Rg3 (100 mmol/L) TRAIL (3 mg/kg) Rg3 (20 mg/kg) |
Sensitized TRAIL-induced cancer cell death CHOP-mediated DR5 upregulation | [87] |
| Sorafenib | In vitro In vivo |
HepG2 Huh7 Five-week-old BALB/c nude mice |
Sorafenib (2.5–20μM) combined with Rg3 (50μg/ml and 100μg /ml) Sorafenib (30 mg/kg) Rg3 (5 mg/kg) |
Sensitized sorafenib-induced cancer cell death by modulating PTEN/AKT signaling pathway | [84] |
| Sorafenib | In vitro | HepG2 Bel7404 |
Sorafenib (5μM) Rg3 (130 μM) |
Relieved the hepatocellular tumor progression through regulating the HK2-mediated glycolysis and PI3K/AKT signaling pathway | [85] |
| Artesunate | In vitro In vivo |
HepG2-SR Male 7-week-old BALB/c-nu/nu mice |
Artesunate (10, 15 mM) Rg3(50, 75 mM) Rg3 (6mg/kg) Artesunate (7.5 mg/kg) Rg3 (12 mg/kg) Artesunate (15 mg/kg) |
Overcoming sorafenib resistance in experimental models, and inhibition of Src/STAT3 signaling and modulation of ROS/STAT3 signaling | [86] |
| Oxaliplatin | In vitro | SMMC-7721 | Oxaliplatin (0.25 μg/ml) Rg3 (15μg/ml) |
Inhibited the proliferation and promoted apoptosis of hepatocellular carcinoma cells via downregulating PCNA and cyclin D1 | [74] |
| Cyclophosphamide | In vivo | Tumor-bearing C57BL/6 mice | Cyclophosphamide (20.0 mg/kg) Rg3 (3.0 mg/kg) |
Increased survival time | [69] |
| TACE | In vitro In vivo |
HepG2 Adult male New Zealand white rabbits |
25-100 mg/l Rg3 (6.0 mg/kg) |
Induced VX2 liver tumor cell apoptosis and inhibited angiogenesis | [91] |
| TACE | In vivo | Male and female Buffalo rats | Rg3 (1 mg/kg) | Effectively limited HCC tumor progression, reduced metastasis, and increased overall survival. | [92] |
| TACE | Clinical Trial | 228 patients with advanced HCC | Rg3 at 20 mg twice a day was administered for 2 months as one course | Prolonged overall survival | [93] |
Furthermore, Rg3 has been found to improve the anti-tumor effect of oxaliplatin-treated liver cancer cells. It may also inhibit the proliferation of HCC and promote cell apoptosis by downregulating PCNA and cyclin D1. These findings suggest that Rg3 could potentially act as a sensitizing agent for radiotherapy and chemotherapy, and work in tandem with oxaliplatin to achieve better results [74]. Similarly, Rg3 decreased cisplatin-induced levels of HO-1/NQO-1 and nuclear Nrf2 in cancer cells with high levels of HO-1/NQO-1 and nuclear Nrf2, thereby increasing the susceptibility of tumor cells to cisplatin [40]. The combination of cyclophosphamide and Rg3 was found to be more effective in suppressing the growth of experimental tumors compared to using only cyclophosphamide therapy or Rg3 alone. Additionally, the occurrence of side effects was significantly lower with this combination treatment. This suggests that the combination therapy may be a safer and more effective option for treating tumors [69]. In addition, Rg3 was showed to be well tolerated and improve the therapeutic effect of TNF-related apoptosis-inducing ligand (TRAIL) in mouse xenograft models. This was achieved through the regulation of death receptor 5 (DR5) and C/EBP homology protein (CHOP) [87]. Furthermore, Rg3 was well tolerated in mice and synergistically inhibited tumor growth of liver cancer xenografts with doxorubicin [88].
Transcatheter arterial chemoembolization (TACE) controls tumor progression and prolongs survival more effectively than conventional cytotoxic chemotherapy in patients with unresectable advanced liver cancer [89]. Although TACE has shown promise in treating tumors, it is limited by a high recurrence rate. This is due to the overexpression of angiogenic and inflammatory factors such as insulin-like growth factor 2 (IGF-2) and VEGF, which promote the metastasis and proliferation of remaining tumor cells [90]. Rg3 combined with TAE effectively reduced overexpression of VEGF and promoted tumor cell apoptosis through a caspase-dependent mechanism [91,92]. According to the clinical trial, the combination of TACE with Rg3 has been shown to increase the median survival time by almost three months in patients with advanced HCC. In addition, the combined use of TACE and Rg3 has been found to enhance the overall survival rate in HCC patients, as compared to those who only received TACE. Moreover, the use of Rg3 in conjunction with TACE can also help to reduce the negative side effects associated with TACE treatment [93].
In conclusion, the combination of Rg3 can overcome drug resistance, synergistically inhibit tumor growth of liver cancer and significantly reduce the occurrence of side effects was in experimental models. In addition, the combination of TACE and Rg3 could enhance the overall survival rate in HCC patients.
3. Liver targeting strategy of Rg3
While Rg3 has shown promise in treating liver disease, its clinical use is limited as its low solubility in both lipids and water. In order for it to be more effective, drugs utilizing Rg3 should be designed to specifically target the liver (Table 3). Therefore, any clinical application of Rg3 should take this into consideration. Novel drug delivery systems (NDDS) such as microemulsions, nano-formulations, liposomes and micelles are popular technologies in the pharmaceutical industry that have shown promise in improving the pharmacokinetic and pharmacodynamic profile of potential drug candidates.
Table 3.
Liver Targeting Strategy of Rg3
| Complex ingredients | Model | Types | Activity and Mechanisms | References |
|---|---|---|---|---|
| Liposomes co-loaded with ursolic acid and Rg3 | In vitro | HepG2 | Affecting apoptosis, cell cycle, and cell proliferation, thereby slowing down the drug release ability in vitro | [94] |
| A nanostructured lipid carrier coated with hyaluronic acid and loaded with oleanolic acid, ursolic acid, and Rg3 | In vivo | Nude mice with tumor | Increased concentrations of the drugs and prolonging their duration in the circulation | [95] |
| A self-micro emulsifying drug delivery system consisting of Rg3, ganoderma lucidum polysaccharide, and oridonin | In vitro | HepG2 Huh7 |
Inhibiting the progression of HCC by targeting multiple pathways and the drug was found to be safe during the acute toxicity tests | [96] |
| Fe@Fe3O4 nanoparticles co-loaded Rg3 | In vivo | Dimethylnitrosamine-induced HCC model | Significantly increased the lifespan of HCC mice in a dimethylnitrosamine-induced HCC model and having a significant inhibitory effect on the development of HCC | [97] |
| Graphene oxide, Rg3, and doxorubicin | In vitro | Huh7 | Significantly reduced the viability of liver cancer cells | [98] |
| The micron- and nano- conjugate linoleic acid vesicles co-loaded Rg3 | / | / | The micron vesicles had a significantly higher loading capacity and encapsulation efficiency compared to the nano- conjugate linoleic acid vesicles. This finding could have positive implications for the field of food and drug delivery science | [99] |
| Lactoferrin co-loaded Rg3 | / | / | Overcomed the non-water-soluble disadvantage of Rg3 | [100] |
Efforts have been made by researchers to prepare liposomes co-loaded with ursolic acid (UA) and Rg3. This preparation has been successful in affecting apoptosis, cell cycle, and cell proliferation, thereby slowing down the drug release ability in vitro [94]. A study has shown the development of HA-OUR-NLC, which is a nanostructured lipid carrier (NLC) coated with hyaluronic acid (HA) and loaded with oleanolic acid (OA), UA, and Rg3. This carrier is designed to accumulate in the tumor site, leading to increased concentrations of the drugs and prolonging their duration in the circulation [95]. In addition, researchers developed a new drug called RGO-SMEDDS, which is a self-micro emulsifying drug delivery system consisting of Rg3, ganoderma lucidum polysaccharide, and oridonin. The drug was found to be effective in inhibiting the progression of HCC by targeting multiple pathways. Moreover, the drug was found to be safe during the acute toxicity tests [96].
In a recent study, researchers developed a new type of nanomedicine by combining Fe@Fe3O4 nanoparticles with ginsenoside Rg3 (NpRg3). This combination resulted in a strong coupling effect. The application of NpRg3 significantly increased the lifespan of HCC mice in a dimethylnitrosamine-induced HCC model. Subsequent research has revealed that NpRg3 has a significant inhibitory effect on the development of HCC and can also eliminate metastases to the lungs. The mechanisms behind NpRg3's anti-HCC properties are thought to be related to systemic biological factors, such as the gut microbiota and host metabolomics [97]. Recent research has shown that conjugates of graphene oxide (GO), Rg3, and doxorubicin (DOX) can significantly reduce the viability of liver cancer cells. This is primarily achieved through the downregulation of transcription regulatory genes and the upregulation of apoptosis genes [98].
Additionally, a study involved the construction of micron- and nano- conjugate linoleic acid vesicles (CLAVs) for the purpose of encapsulating and releasing ginsenoside Rg3. The researchers found that the micron vesicles had a significantly higher loading capacity and encapsulation efficiency compared to the nano-CLAVs. This finding could have positive implications for the field of food and drug delivery science [99]. In addition, another study utilized Rg3 to interact with bovine lactoferrin (LF), resulting in the formation of a water-dispersed LF-Rg3 complex. Additionally, a highly stable LF-Rg3 oil-in-water emulsion was prepared, which overcomes the non-water-soluble disadvantage of Rg3. These findings expand the potential biological applications of the LF-Rg3 complex, particularly in the field of food healthcare [100].
There is currently limited information available regarding Rg3 drug delivery systems for liver disease therapy. However, considering the potential therapeutic benefits of Rg3, it is important to further explore appropriate carrier systems that can effectively deliver Rg3 to alleviate the progression of liver diseases.
4. Conclusions and perspectives
Liver diseases are a significant global health concern, with numerous factors such as viruses, drugs, and obesity contributing to their development. These diseases are characterized by various mechanisms, including inflammation, apoptosis, autophagy, oxidative stress, and fibrosis. Unfortunately, current drug treatments are not optimal, and there is a need for new agents to effectively treat liver diseases. This review explores the role of Rg3, the primary chemical component and pharmacological active compound of ginsenosides, in liver protection for various liver diseases. The study finds that Rg3 inhibits viral proliferation by stimulating P21/CDK1/Drp1 and TRAF6/TAK1 degradation while also inhibiting the JNK/AP-1 signaling pathway. Rg3 can also reduce acute liver injury and the underlying mechanisms are related to the anti-inflammatory, antioxidant, autophagy and apoptosis-regulatory effects. In addition, Rg3 ameliorates NAFLD through mechanisms including regulation of lipid metabolism and its anti-inflammatory effects. Rg3 exhibits potential hepatoprotection effects against liver fibrosis through multiple mechanisms associated with PI3K/AKT-mediated inflammation and autophagy. Furthermore, Rg3 is a promising candidate in the treatment of HCC with multiple potential mechanisms, including inhibition of proliferation, promotion of immunity, and reduction of angiogenesis, migration and invasion, induction of apoptosis and autophagy in HCC cells.
The challenges facing Rg3 transformation research are primarily related to its small molecular weight, poor solubility, and limited absorbability, which ultimately restrict its application. To overcome these challenges, there is an urgent need for the development of new technologies and agents that can enhance the bio-utilization of Rg3 and facilitate the creation of more effective drugs for the clinical treatment of liver diseases. While Rg3 has shown potential therapeutic efficacy in various liver diseases, there is limited research on its role in alcoholic and autoimmune liver diseases. Current studies primarily focus on its anti-hepatic injury and anti-liver cancer effects in liver diseases. Liver fibrosis is a crucial pathway in the development of various liver diseases. Its potential for reversibility makes it especially important to study. However, there is currently a lack of research in this area, indicating a need for further investigation.
In future research, a multi-omics approach (such as proteomics, transcriptomics, and metabolomics) can be utilized to identify differentially expressed genes, proteins, and metabolites, elucidating the specific molecular mechanisms and targets of Rg3 in its hepatoprotective effects. Additionally, combining Rg3 with other drugs may serve as a promising strategy to improve the therapeutic potential of Rg3. Currently, the combined applications of Rg3 are primarily focused on treating liver cancer. However, further research is necessary to determine its efficacy in treating other liver diseases. To enhance the synergistic effects of Rg3, high-throughput screening and network pharmacology can be used to identify suitable combination strategies. As the current evidence on the effects of Rg3 in liver diseases is primarily based on cell lines and animal models, further clinical trials are necessary to investigate its clinical effects. However, we anticipate that Rg3 will prove to be a safe and beneficial product for consumers' health in the near future.
Funding
This study was sponsored by the Shanghai Science and Technology Plan Project (23010504200); the "Shuguang Program" (20SG50) funded by Shanghai Education Development Foundation and Shanghai Municipale Education Commission; the Shanghai Talent Development Fund (2020125); the Key Lab of Exercise and Health Sciences of Ministry of Education (Shanghai University of Sport) (2022KF001); and the Shanghai Key Lab of Human Performance (Shanghai University of Sport) (NO. 11DZ2261100).
CRediT authorship contribution statement
Wenhong Wang: Conceptualization, Investigation, Software, Writing – original draft. Ke Li: Writing − review & editing, Drawing pictures. Weihua Xiao: Funding, Data curation, Supervision, Visualization.
Declaration of competing interest
The authors declare no conflict of interest.
Contributor Information
Wenhong Wang, Email: 13595606803@163.com.
Ke Li, Email: ley_like@163.com.
Weihua Xiao, Email: xiao_weihua@163.com.
References
- 1.Xiao J., Wang F., Wong N.K., He J., Zhang R., Sun R., Xu Y., Liu Y., Li W., Koike K., et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019;71(1):212–221. doi: 10.1016/j.jhep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Neshat S.Y., Quiroz V.M., Wang Y., Tamayo S., Doloff J.C. Liver disease: induction, progression, immunological mechanisms, and therapeutic interventions. Int J Mol Sci. 2021;22(13) doi: 10.3390/ijms22136777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J., Wang X., Xia T., Bi Y., Liu B., Fu J., Zhu R. Molecular mechanisms and therapeutic implications of dihydromyricetin in liver disease. Biomed Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.111927. [DOI] [PubMed] [Google Scholar]
- 5.Wang C., Ma C., Gong L., Dai S., Li Y. Preventive and therapeutic role of betaine in liver disease: a review on molecular mechanisms. Eur J Pharmacol. 2021;912 doi: 10.1016/j.ejphar.2021.174604. [DOI] [PubMed] [Google Scholar]
- 6.Xiao X., Hu Q., Deng X., Shi K., Zhang W., Jiang Y., Ma X., Zeng J., Wang X. Old wine in new bottles: kaempferol is a promising agent for treating the trilogy of liver diseases. Pharmacol Res. 2021;175 doi: 10.1016/j.phrs.2021.106005. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q.H., Wu C.F., Duan L., Yang J.Y. Protective effects of ginsenoside Rg(3) against cyclophosphamide-induced DNA damage and cell apoptosis in mice. Arch Toxicol. 2008;82(2):117–123. doi: 10.1007/s00204-007-0224-3. [DOI] [PubMed] [Google Scholar]
- 8.Lü J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Current Vascular Pharmacol. 2009;7(3):293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms S. Cancer prevention and therapeutics: Panax ginseng, Alternative medicine review. J Clinical Therapeut. 2004;9(3):259–274. [PubMed] [Google Scholar]
- 10.Kim I.W., Sun W.S., Yun B.S., Kim N.R., Min D., Kim S.K. Characterizing a full spectrum of physico-chemical properties of (20S)- and (20R)-ginsenoside Rg3 to be proposed as standard reference materials. J Ginseng Res. 2013;37(1):124–134. doi: 10.5142/jgr.2013.37.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W.C., Huang T.H., Yeh K.W., Chen Y.L., Shen S.C., Liou C.J. Ginsenoside Rg3 ameliorates allergic airway inflammation and oxidative stress in mice. J Ginseng Res. 2021;45(6):654–664. doi: 10.1016/j.jgr.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma C.H., Chou W.C., Wu C.H., Jou I.M., Tu Y.K., Hsieh P.L., Tsai K.L. Ginsenoside Rg3 attenuates TNF-α-induced damage in chondrocytes through regulating SIRT1-mediated anti-apoptotic and anti-inflammatory mechanisms. Antioxidants (Basel) 2021;10(12) doi: 10.3390/antiox10121972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., Liu T., Li W., Li J., Wang C., Zhang K. Insights into the antitumor mechanism of ginsenosides Rg3. Mol Biol Rep. 2021;48(3):2639–2652. doi: 10.1007/s11033-021-06187-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W., Aryan M., Qian S., Cabrera R., Liu X. A focused review on recent advances in the diagnosis and treatment of viral hepatitis. Gastroenterol Res. 2021;14(3):139–156. doi: 10.14740/gr1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisano M.B., Giadans C.G., Flichman D.M., Re V.E., Preciado M.V., Valva P. Viral hepatitis update: progress and perspectives. World J Gastroenterol. 2021;27(26):4018–4044. doi: 10.3748/wjg.v27.i26.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrscher C., Roingeard P., Blanchard E. Hepatitis B virus entry into cells. Cells. 2020;9(6) doi: 10.3390/cells9061486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J., Tsai K.-N., Ou J.-h.J. Mechanisms of hepatitis B virus-induced hepatocarcinogenesis. Viruses and Human Cancer. 2021:47–70. doi: 10.1007/978-3-030-57362-1_3. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y. Hepatitis B virus-associated hepatocellular carcinoma. Adv Exp Med Biol. 2017;1018:11–21. doi: 10.1007/978-981-10-5765-6_2. [DOI] [PubMed] [Google Scholar]
- 19.Zhao F., Xie X., Tan X., Yu H., Tian M., Lv H., Qin C., Qi J., Zhu Q. The functions of hepatitis B virus encoding proteins: viral persistence and liver pathogenesis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.691766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang L.J., Choi Y.J., Lee S.G. Stimulation of TRAF6/TAK1 degradation and inhibition of JNK/AP-1 signalling by ginsenoside Rg3 attenuates hepatitis B virus replication. Int J Biochem Cell Biol. 2013;45(11):2612–2621. doi: 10.1016/j.biocel.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Roger S., Ducancelle A., Le Guillou-Guillemette H., Gaudy C., Lunel F. HCV virology and diagnosis. Clinics Res Hepatol Gastroenterol. 2021;45(3) doi: 10.1016/j.clinre.2021.101626. [DOI] [PubMed] [Google Scholar]
- 22.Rabaan A.A., Al-Ahmed S.H., Bazzi A.M., Alfouzan W.A., Alsuliman S.A., Aldrazi F.A., Haque S. Overview of hepatitis C infection, molecular biology, and new treatment. J Infect Public Health. 2020;13(5):773–783. doi: 10.1016/j.jiph.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Hino K., Nishina S., Sasaki K., Hara Y. Mitochondrial damage and iron metabolic dysregulation in hepatitis C virus infection. Free Radic Biol Med. 2019;133:193–199. doi: 10.1016/j.freeradbiomed.2018.09.044. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.J., Jang J.Y., Kim E.J., Cho E.K., Ahn D.G., Kim C., Park H.S., Jeong S.W., Lee S.H., Kim S.G., et al. Ginsenoside Rg3 restores hepatitis C virus-induced aberrant mitochondrial dynamics and inhibits virus propagation. Hepatology. 2017;66(3):758–771. doi: 10.1002/hep.29177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo Y.C., Lee J., Park S.R., Nam K.Y., Cho Y.H., Choi J.E. Protective effect of ginsenoside-Rb2 from Korean red ginseng on the lethal infection of haemagglutinating virus of Japan in mice. J Ginseng Res. 2013;37(1):80–86. doi: 10.5142/jgr.2013.37.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katarey D., Verma S. Drug-induced liver injury. Clinical Medicine (London, England) 2016;16(Suppl 6):s104–s109. doi: 10.7861/clinmedicine.16-6s-s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davern T.J. Drug-induced liver disease. Clin Liver Dis. 2012;16(2):231–245. doi: 10.1016/j.cld.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Gum S.I., Cho M.K. Korean red ginseng extract prevents APAP-induced hepatotoxicity through metabolic enzyme regulation: the role of ginsenoside Rg3, a protopanaxadiol. Liver Int. 2013;33(7):1071–1084. doi: 10.1111/liv.12046. [DOI] [PubMed] [Google Scholar]
- 29.Gum S.I., Cho M.K. The amelioration of N-acetyl-p-benzoquinone imine toxicity by ginsenoside Rg3: the role of Nrf2-mediated detoxification and Mrp1/Mrp3 transports. Oxid Med Cell Longev. 2013;2013 doi: 10.1155/2013/957947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y.D., Hou J.G., Liu W., Ren S., Wang Y.P., Zhang R., Chen C., Wang Z., Li W. 20(R)-ginsenoside Rg3, a rare saponin from red ginseng, ameliorates acetaminophen-induced hepatotoxicity by suppressing PI3K/AKT pathway-mediated inflammation and apoptosis. Int Immunopharmacol. 2018;59:21–30. doi: 10.1016/j.intimp.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Zhu C.L., Yao R.Q., Li L.X., Li P., Xie J., Wang J.F., Deng X.M. Mechanism of mitophagy and its role in sepsis induced organ dysfunction: a review. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.664896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strnad P., Tacke F., Koch A., Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14(1):55–66. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 33.Yin X., Xin H., Mao S., Wu G., Guo L. The role of autophagy in sepsis: protection and injury to organs. Front Physiol. 2019;10:1071. doi: 10.3389/fphys.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xing W., Yang L., Peng Y., Wang Q., Gao M., Yang M., Xiao X. Ginsenoside Rg3 attenuates sepsis-induced injury and mitochondrial dysfunction in liver via AMPK-mediated autophagy flux. Biosci Rep. 2017;37(4) doi: 10.1042/BSR20170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu P., Yu X., Peng Y., Wang Q.L., Deng L.T., Xing W. Ginsenoside Rg3 alleviates septic liver injury by regulating the lncRNA TUG1/miR-200c-3p/SIRT1 axis. J Inflamm (Lond) 2021;18(1):31. doi: 10.1186/s12950-021-00296-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Azman K.F., Safdar A., Zakaria R. D-galactose-induced liver aging model: its underlying mechanisms and potential therapeutic interventions. Exp Gerontol. 2021;150 doi: 10.1016/j.exger.2021.111372. [DOI] [PubMed] [Google Scholar]
- 37.Li W., Wang J.Q., Zhou Y.D., Hou J.G., Liu Y., Wang Y.P., Gong X.J., Lin X.H., Jiang S., Wang Z. Rare ginsenoside 20(R)-Rg3 inhibits D-galactose-induced liver and kidney injury by regulating oxidative stress-induced apoptosis. Am J Chin Med. 2020;48(5):1141–1157. doi: 10.1142/S0192415X20500561. [DOI] [PubMed] [Google Scholar]
- 38.Romani A.M.P. Cisplatin in cancer treatment. Biochem Pharmacol. 2022;206 doi: 10.1016/j.bcp.2022.115323. [DOI] [PubMed] [Google Scholar]
- 39.Quintanilha J.C.F., Saavedra K.F., Visacri M.B., Moriel P., Salazar L.A. Role of epigenetic mechanisms in cisplatin-induced toxicity. Critical Rev Oncol/hematol. 2019;137:131–142. doi: 10.1016/j.critrevonc.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Lee C.K., Park K.K., Chung A.S., Chung W.Y. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food Chem Toxicol. 2012;50(7):2565–2574. doi: 10.1016/j.fct.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Park H.M., Kim S.J., Mun A.R., Go H.K., Kim G.B., Kim S.Z., Jang S.I., Lee S.J., Kim J.S., Kang H.S. Korean red ginseng and its primary ginsenosides inhibit ethanol-induced oxidative injury by suppression of the MAPK pathway in TIB-73 cells. J Ethnopharmacol. 2012;141(3):1071–1076. doi: 10.1016/j.jep.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 42.Lee H.U., Bae E.A., Han M.J., Kim D.H. Hepatoprotective effect of 20(S)-ginsenosides Rg3 and its metabolite 20(S)-ginsenoside Rh2 on tert-butyl hydroperoxide-induced liver injury. Biol Pharm Bull. 2005;28(10):1992–1994. doi: 10.1248/bpb.28.1992. [DOI] [PubMed] [Google Scholar]
- 43.Kang K.S., Kim H.Y., Yamabe N., Park J.H., Yokozawa T. Preventive effect of 20(S)-ginsenoside Rg3 against lipopolysaccharide-induced hepatic and renal injury in rats. Free Radic Res. 2007;41(10):1181–1188. doi: 10.1080/10715760701581740. [DOI] [PubMed] [Google Scholar]
- 44.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nature Medicine. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masoodi M., Gastaldelli A., Hyötyläinen T., Arretxe E., Alonso C., Gaggini M., et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18(12):835–856. doi: 10.1038/s41575-021-00502-9. [DOI] [PubMed] [Google Scholar]
- 46.Estes C., Anstee Q.M., Arias-Loste M.T., Bantel H., Bellentani S., Caballeria J., Colombo M., Craxi A., Crespo J., Day C.P., et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 47.Foerster F., Gairing S.J., Muller L., Galle P.R. NAFLD-driven HCC: safety and efficacy of current and emerging treatment options. J Hepatol. 2022;76(2):446–457. doi: 10.1016/j.jhep.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Lee S., Lee M.S., Kim C.T., Kim I.H., Kim Y. Ginsenoside Rg3 reduces lipid accumulation with AMP-Activated Protein Kinase (AMPK) activation in HepG2 cells. Int J Mol Sci. 2012;13(5):5729–5739. doi: 10.3390/ijms13055729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J.B., Yoon S.J., Lee S.H., Lee M.S., Jung H., Kim T.D., Yoon S.R., Choi I., Kim I.S., Chung S.W., et al. Ginsenoside Rg3 ameliorated HFD-induced hepatic steatosis through downregulation of STAT5-PPARgamma. J Endocrinol. 2017;235(3):223–235. doi: 10.1530/JOE-17-0233. [DOI] [PubMed] [Google Scholar]
- 50.Nan B., Liu Y.L., You Y., Li W.C., Fan J.J., Wang Y.S., Piao C.H., Hu D.L., Lu G.J., Wang Y.H. Protective effects of enhanced minor ginsenosides in Lactobacillus fermentum KP-3-fermented ginseng in mice fed a high fat diet. Food Funct. 2018;9(11):6020–6028. doi: 10.1039/c8fo01056k. [DOI] [PubMed] [Google Scholar]
- 51.Arrese M., Cabrera D., Kalergis A.M., Feldstein A.E. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61(5):1294–1303. doi: 10.1007/s10620-016-4049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maceyka M., Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510(7503):58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J.C., Jeon J.Y., Yang W.S., Kim C.H., Eom D.W. Combined amelioration of ginsenoside (Rg1, Rb1, and Rg3)-enriched Korean red ginseng and probiotic lactobacillus on non-alcoholic fatty liver disease. Current Pharmaceut Biotechnol. 2019;20(3):222–231. doi: 10.2174/1389201020666190311143554. [DOI] [PubMed] [Google Scholar]
- 54.Choi S.Y., Park J.S., Shon C.H., Lee C.Y., Ryu J.M., Son D.J., Hwang B.Y., Yoo H.S., Cho Y.C., Lee J., et al. Fermented Korean red ginseng extract enriched in rd and Rg3 protects against non-alcoholic fatty liver disease through regulation of mTORC1. Nutrients. 2019;11(12) doi: 10.3390/nu11122963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J.H., Oh J.Y., Kim S.H., Oh I.J., Lee Y.H., Lee K.W., Lee W.H., Kim J.H. Pharmaceutical efficacy of gypenoside LXXV on non-alcoholic steatohepatitis (NASH) Biomolecules. 2020;10(10) doi: 10.3390/biom10101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aydın M.M., Akçalı K.C. Liver fibrosis. Turk J Gastroenterol. 2018;29(1):14–21. doi: 10.5152/tjg.2018.17330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Khomich O., Ivanov A.V., Bartosch B. Metabolic hallmarks of hepatic stellate cells in liver fibrosis. Cells. 2019;9(1) doi: 10.3390/cells9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li K., Wang W., Xiao W. Astaxanthin: a promising therapeutic agent for organ fibrosis. Pharmacol Res. 2023;188 doi: 10.1016/j.phrs.2023.106657. [DOI] [PubMed] [Google Scholar]
- 60.Acharya P., Chouhan K., Weiskirchen S., Weiskirchen R. Cellular mechanisms of liver fibrosis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.671640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dewidar B., Meyer C., Dooley S., Meindl-Beinker A.N. TGF-Β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells. 2019;8(11) doi: 10.3390/cells8111419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui X., Wang K., Zhang J., Cao Z.B. Aerobic Exercise ameliorates myocardial fibrosis via affecting vitamin D receptor and transforming growth factor-beta1 signaling in vitamin D-deficient mice. Nutrients. 2023;15(3) doi: 10.3390/nu15030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X., Mi X., Wang Z., Zhang M., Hou J., Jiang S., Wang Y., Chen C., Li W. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis. 2020;11(6):454. doi: 10.1038/s41419-020-2597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA: A Cancer J Clinicians. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 65.Wang N., Wang S., Li M.Y., Hu B.G., Liu L.P., Yang S.L., Yang S., Gong Z., Lai P.B.S., Chen G.G. Cancer stem cells in hepatocellular carcinoma: an overview and promising therapeutic strategies. Therapeut Adv Med Oncol. 2018;10 doi: 10.1177/1758835918816287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Matteis S., Ragusa A., Marisi G., De Domenico S., Casadei Gardini A., Bonafè M., Giudetti A.M. Aberrant metabolism in hepatocellular carcinoma provides diagnostic and therapeutic opportunities. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/7512159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C., Liu L., Yu Y., Chen B., Tang C., Li X. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Mol Med Rep. 2012;5(5):1295–1298. doi: 10.3892/mmr.2012.808. [DOI] [PubMed] [Google Scholar]
- 68.Park H.M., Kim S.J., Kim J.S., Kang H.S. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food Chem Toxicol. 2012;50(8):2736–2741. doi: 10.1016/j.fct.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 69.Jiang J.W., Chen X.M., Chen X.H., Zheng S.S. Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via intrinsic apoptotic pathway. World J Gastroenterol. 2011;17(31):3605–3613. doi: 10.3748/wjg.v17.i31.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheong J.H., Kim H., Hong M.J., Yang M.H., Kim J.W., Yoo H., Yang H., Park J.H., Sung S.H., Kim H.P., et al. Stereoisomer-specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: disparity in cytotoxicity and autophagy-inducing effects due to 20(S)-epimers. Biol Pharm Bull. 2015;38(1):102–108. doi: 10.1248/bpb.b14-00603. [DOI] [PubMed] [Google Scholar]
- 71.Wu R., Ru Q., Chen L., Ma B., Li C. Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice. J Food Sci. 2014;79(7):H1430–H1435. doi: 10.1111/1750-3841.12518. [DOI] [PubMed] [Google Scholar]
- 72.Teng S., Wang Y., Li P., Liu J., Wei A., Wang H., Meng X., Pan D., Zhang X. Effects of R type and S type ginsenoside Rg3 on DNA methylation in human hepatocarcinoma cells. Mol Med Rep. 2017;15(4):2029–2038. doi: 10.3892/mmr.2017.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X., Tsauo J., Geng C., Zhao H., Lei X., Li X. Ginsenoside Rg3 decreases NHE1 expression via inhibiting EGF-EGFR-ERK1/2-HIF-1 alpha pathway in hepatocellular carcinoma: a novel antitumor mechanism. Am J Chin Med. 2018;46(8):1915–1931. doi: 10.1142/S0192415X18500969. [DOI] [PubMed] [Google Scholar]
- 74.Shan K., Wang Y., Hua H., Qin S., Yang A., Shao J. Ginsenoside Rg3 combined with oxaliplatin inhibits the proliferation and promotes apoptosis of hepatocellular carcinoma cells via downregulating PCNA and cyclin D1. Biological Pharmaceut Bulletin. 2019;42(6):900–905. doi: 10.1248/bpb.b18-00852. [DOI] [PubMed] [Google Scholar]
- 75.Siegel R., Ward E., Brawley O., Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: A Cancer J Clinicians. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 76.Yuan C., Ning Y., Pan Y. Emerging roles of HOTAIR in human cancer. J Cell Biochem. 2020;121(5–6):3235–3247. doi: 10.1002/jcb.29591. [DOI] [PubMed] [Google Scholar]
- 77.Pu Z., Ge F., Wang Y., Jiang Z., Zhu S., Qin S., Dai Q., Liu H., Hua H. Ginsenoside-Rg3 inhibits the proliferation and invasion of hepatoma carcinoma cells via regulating long non-coding RNA HOX antisense intergenic. Bioengineered. 2021;12(1):2398–2409. doi: 10.1080/21655979.2021.1932211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang H., Tang Q.F., Sun M.Y., Zhang C.Y., Zhu J.Y., Shen Y.L., Zhao B., Shao Z.Y., Zhang L.J., Zhang H. ARHGAP9 suppresses the migration and invasion of hepatocellular carcinoma cells through up-regulating FOXJ2/E-cadherin. Cell Death Dis. 2018;9(9):916. doi: 10.1038/s41419-018-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun M.Y., Song Y.N., Zhang M., Zhang C.Y., Zhang L.J., Zhang H. Ginsenoside Rg3 inhibits the migration and invasion of liver cancer cells by increasing the protein expression of ARHGAP9. Oncol Lett. 2019;17(1):965–973. doi: 10.3892/ol.2018.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu S., Zhu Y., Xia X., Xu X., Chen F., Miao X., Chen X. Ginsenoside Rg3 prolongs survival of the orthotopic hepatocellular carcinoma model by inducing apoptosis and inhibiting angiogenesis. Anal Cell Pathol (Amst) 2019;2019 doi: 10.1155/2019/3815786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen K.F., Chen H.L., Tai W.T., Feng W.C., Hsu C.H., Chen P.J., Cheng A.L. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Experiment Therapeut. 2011;337(1):155–161. doi: 10.1124/jpet.110.175786. [DOI] [PubMed] [Google Scholar]
- 82.Fornari F., Giovannini C., Piscaglia F., Gramantieri L. Elucidating the molecular basis of sorafenib resistance in HCC: current findings and future directions. J Hepatocell Carcinoma. 2021;8:741–757. doi: 10.2147/JHC.S285726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W., Xiao Y., Li S., Zhu X., Meng L., Song C., Yu C., Jiang N., Liu Y. Synergistic activity of magnolin combined with B-RAF inhibitor SB590885 in hepatocellular carcinoma cells via targeting PI3K-AKT/mTOR and ERK MAPK pathway. American J Translat Res. 2019;11(6):3816–3824. [PMC free article] [PubMed] [Google Scholar]
- 84.Lu M., Fei Z., Zhang G. Synergistic anticancer activity of 20(S)-Ginsenoside Rg3 and Sorafenib in hepatocellular carcinoma by modulating PTEN/Akt signaling pathway. Biomed Pharmacother. 2018;97:1282–1288. doi: 10.1016/j.biopha.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 85.Wei Q., Ren Y., Zheng X., Yang S., Lu T., Ji H., Hua H., Shan K. Ginsenoside Rg3 and sorafenib combination therapy relieves the hepatocellular carcinomaprogression through regulating the HK2-mediated glycolysis and PI3K/Akt signaling pathway. Bioengineered. 2022;13(5):13919–13928. doi: 10.1080/21655979.2022.2074616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y.J., Wu J.Y., Deng Y.Y., Wu Y., Wang X.Q., Li A.S., Wong L.Y., Fu X.Q., Yu Z.L., Liang C. Ginsenoside Rg3 in combination with artesunate overcomes sorafenib resistance in hepatoma cell and mouse models. J Ginseng Res. 2022;46(3):418–425. doi: 10.1016/j.jgr.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J.Y., Jung K.H., Morgan M.J., Kang Y.R., Lee H.S., Koo G.B., Hong S.S., Kwon S.W., Kim Y.S. Sensitization of TRAIL-induced cell death by 20(S)-ginsenoside Rg3 via CHOP-mediated DR5 upregulation in human hepatocellular carcinoma cells. Mol Cancer Ther. 2013;12(3):274–285. doi: 10.1158/1535-7163.MCT-12-0054. [DOI] [PubMed] [Google Scholar]
- 88.Kim D.G., Jung K.H., Lee D.G., Yoon J.H., Choi K.S., Kwon S.W., Shen H.M., Morgan M.J., Hong S.S., Kim Y.S. 20(S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget. 2014;5(12):4438–4451. doi: 10.18632/oncotarget.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamanaka K., Hatano E., Kitamura K., Iida T., Ishii T., Machimito T., Taura K., Yasuchika K., Isoda H., Shibata T., et al. Early evaluation of transcatheter arterial chemoembolization-refractory hepatocellular carcinoma. J Gastroenterol. 2012;47(3):343–346. doi: 10.1007/s00535-011-0511-x. [DOI] [PubMed] [Google Scholar]
- 90.Huo Y.R., Eslick G.D. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncology. 2015;1(6):756–765. doi: 10.1001/jamaoncol.2015.2189. [DOI] [PubMed] [Google Scholar]
- 91.Yu Y., Zhang C., Liu L., Li X. Hepatic arterial administration of ginsenoside Rg3 and transcatheter arterial embolization for the treatment of VX2 liver carcinomas. Exp Ther Med. 2013;5(3):761–766. doi: 10.3892/etm.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou B., Wang J., Yan Z. Ginsenoside Rg3 attenuates hepatoma VEGF overexpression after hepatic artery embolization in an orthotopic transplantation hepatocellular carcinoma rat model. OncoTargets and Therapy. 2014;7:1945–1954. doi: 10.2147/OTT.S69830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou B., Yan Z., Liu R., Shi P., Qian S., Qu X., Zhu L., Zhang W., Wang J. Prospective study of transcatheter arterial chemoembolization (TACE) with ginsenoside Rg3 versus TACE alone for the treatment of patients with advanced hepatocellular carcinoma. Radiology. 2016;280(2):630–639. doi: 10.1148/radiol.2016150719. [DOI] [PubMed] [Google Scholar]
- 94.Wang B., Xu Q., Zhou C., Lin Y. Liposomes co-loaded with ursolic acid and ginsenoside Rg3 in the treatment of hepatocellular carcinoma. Acta Biochim Pol. 2021;68(4):711–715. doi: 10.18388/abp.2020_5608. [DOI] [PubMed] [Google Scholar]
- 95.Sun S., Guan Q., Shang E., Xiao H., Yu X., Shi L., Zhao C., Guo Y., Lv S., Li Y. Hyaluronic acid-coated nanostructured lipid carriers for loading multiple traditional Chinese medicine components for liver cancer treatment. Pakistan J Pharmaceut Sci. 2020;33(1):109–119. [PubMed] [Google Scholar]
- 96.He S., Tian S., He X., Le X., Ning Y., Chen J., Chen H., Mu J., Xu K., Xiang Q., et al. Multiple targeted self-emulsifying compound RGO reveals obvious anti-tumor potential in hepatocellular carcinoma. Mol Ther Oncolytics. 2021;22:604–616. doi: 10.1016/j.omto.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ren Z., Chen X., Hong L., Zhao X., Cui G., Li A., Liu Y., Zhou L., Sun R., Shen S., et al. Nanoparticle conjugation of ginsenoside Rg3 inhibits hepatocellular carcinoma development and metastasis. Small. 2020;16(2) doi: 10.1002/smll.201905233. [DOI] [PubMed] [Google Scholar]
- 98.Rahimi S., van Leeuwen D., Roshanzamir F., Pandit S., Shi L., Sasanian N., Nielsen J., Esbjorner E.K., Mijakovic I. Ginsenoside Rg3 reduces the toxicity of graphene oxide used for pH-responsive delivery of doxorubicin to liver and breast cancer cells. Pharmaceutics. 2023;15(2) doi: 10.3390/pharmaceutics15020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu H., Hu X., Li L., Meng X., Fang Y., Xia Y. Micron and nano hybrid ufasomes from conjugated linoleic acid, their vesiculation and encapsulation of ginsenoside Rg3. J Sci Food Agric. 2022;102:4140–4150. doi: 10.1002/jsfa.11763. [DOI] [PubMed] [Google Scholar]
- 100.Yan M., Diao M., Zhang C., Shen X., Zhan X., Xi C., Zhao C., Zhang T. Lactoferrin-ginsenoside Rg3 complex ingredients: study of interaction mechanism and preparation of oil-in-water emulsion. Food Chem. 2021;363 doi: 10.1016/j.foodchem.2021.130239. [DOI] [PubMed] [Google Scholar]