Abstract
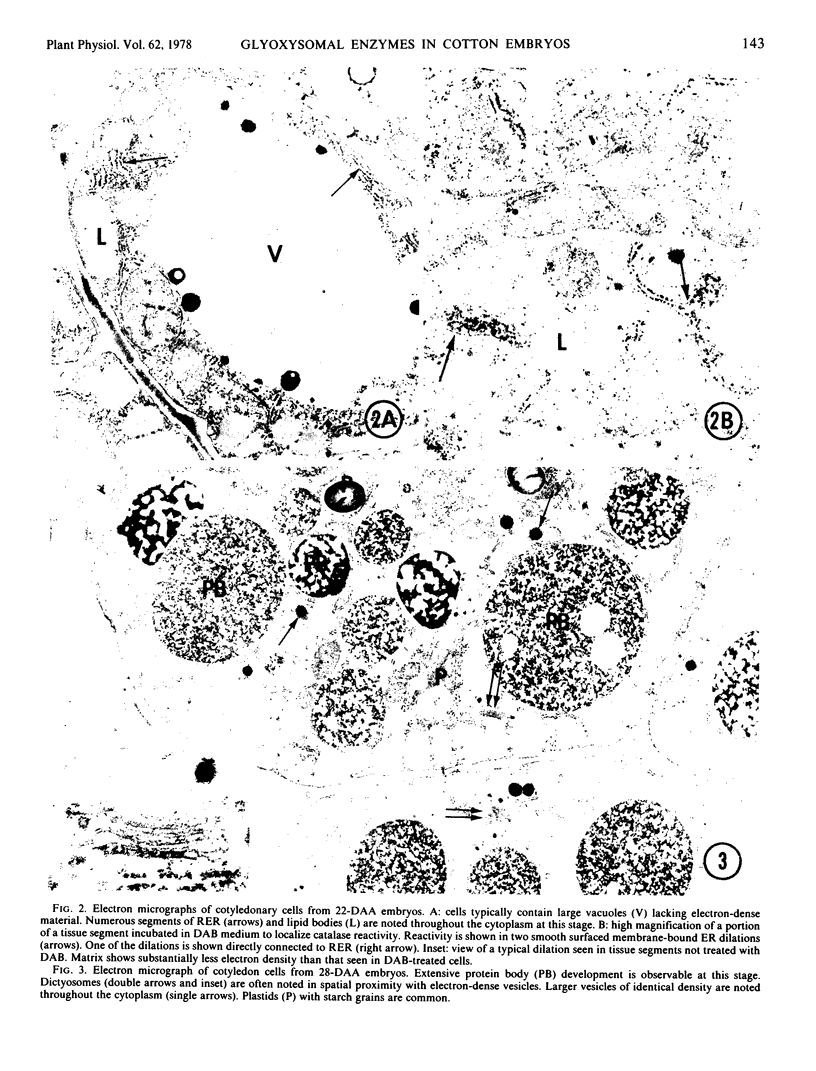
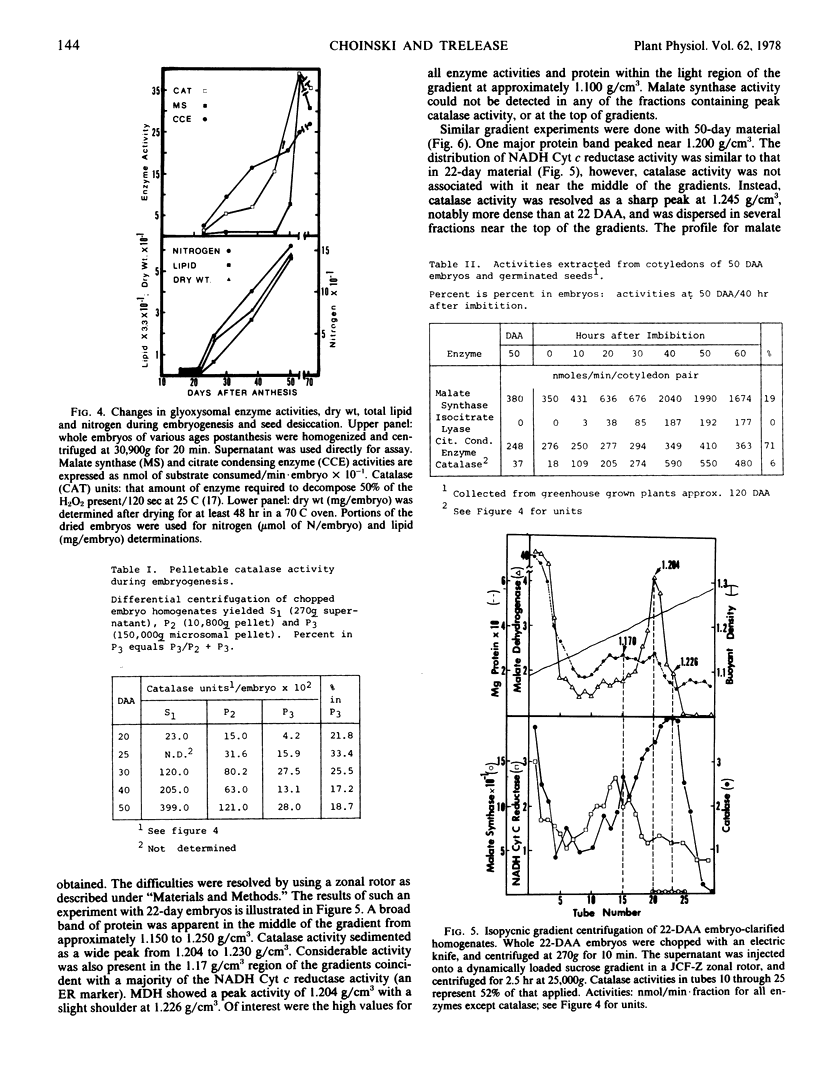
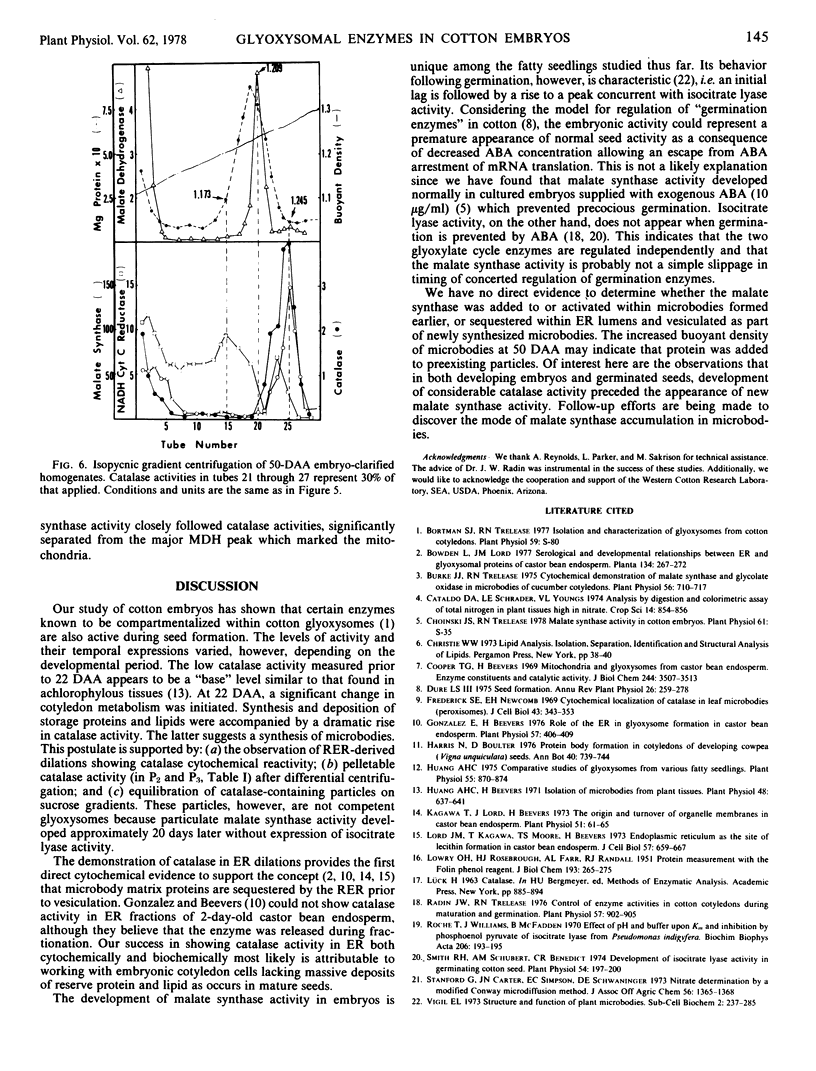
The sequence of glyoxysomal enzyme development was investigated in cotyledons of cotton (Gossypium hirsutum L. cv. Deltapine 16) embryos from 16 to 70 days after anthesis (DAA). Catalase, malate dehydrogenase, and citrate condensing enzyme activities were barely detectable prior to 22 DAA, but showed dramatic increases from 22 to 50 DAA. Development of malate synthase activity, however, was delayed during this period, rising to peak activity from 45 to 50 DAA (just prior to desiccation) in the absence of any detectable isocitrate lyase activity. Substantial activities of all of these enzymes (except isocitrate lyase) persisted in the dry seeds. Isopycnic centrifugations on sucrose gradients demonstrated that the enzymes were compartmentalized within particles increasing in buoyant density with time of development (1.226 to 1.245 grams per cubic centimeter from 22 to 50 DAA). Of particular significance were the observations in 22-day embryos of smooth surfaced membrane dilations of rough endoplasmic reticulum having cytochemical catalase reactivity, and the demonstrations of catalase activities in microsomal fractions isolated throughout the 16- to 50-DAA period. Our data do not allow determination of the mechanism(s) for enzyme activation and/or addition to previously existing or newly formed microbodies, but do show that development and acquisition of enzyme activities within glyoxysomes occur sequentially and thus are not regulated in concert as previously thought.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. J., Trelease R. N. Cytochemical demonstration of malate synthase and glycolate oxidase in microbodies of cucumber cotyledons. Plant Physiol. 1975 Nov;56(5):710–717. doi: 10.1104/pp.56.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Frederick S. E., Newcomb E. H. Cytochemical localization of catalase in leaf microbodies (peroxisomes). J Cell Biol. 1969 Nov;43(2):343–353. doi: 10.1083/jcb.43.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E., Beevers H. Role of the endoplasmic reticulum in glyoxysome formation in castor bean endosperm. Plant Physiol. 1976 Mar;57(3):406–409. doi: 10.1104/pp.57.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H. Comparative studies of glyoxysomes from various Fatty seedlings. Plant Physiol. 1975 May;55(5):870–874. doi: 10.1104/pp.55.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W. Control of Enzyme Activities in Cotton Cotyledons during Maturation and Germination: I. Nitrate Reductase and Isocitrate Lyase. Plant Physiol. 1976 Jun;57(6):902–905. doi: 10.1104/pp.57.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche T. E., Williams J. O., McFadden B. A. Effect of pH and buffer upon Km and inhibition by phosphoenolpyruvate of isocitrate lyase from Pseudomonas indigofera. Biochim Biophys Acta. 1970 Apr 22;206(1):193–195. doi: 10.1016/0005-2744(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Smith R. H., Schubert A. M., Benedict C. R. The development of isocitric lyase activity in germinating cotton seed. Plant Physiol. 1974 Aug;54(2):197–200. doi: 10.1104/pp.54.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Structure and function of plant microbodies. Subcell Biochem. 1973;2(3):237–285. [PubMed] [Google Scholar]





