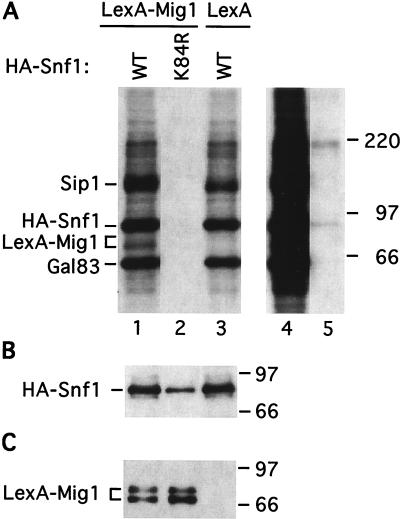
FIG. 4.
Snf1-dependent phosphorylation of Mig1 in vitro. Strain FY250 (wild type [WT]) was transformed with plasmids expressing HA-Snf1 or HA-Snf1K84R and LexA-Mig1 or the LexA moiety (from the parental vector). Protein extracts were prepared from cells grown in selective SC medium plus 2% glucose; previous studies have shown that the Snf1 kinase is activated during preparation of the extracts (13, 58). (A and B) Proteins (200 μg) were immunoprecipitated with anti-HA antibody. (A) Half of the immunoprecipitate was resuspended in kinase assay buffer and incubated with [γ-32P]ATP. Proteins were then separated by SDS-PAGE in 8% polyacrylamide, and the gel was subjected to autoradiography to detect phosphorylated products. The left panel (lanes 1 to 3) shows a 3.5-h exposure, and the right panel (lanes 4 and 5) shows a 15-h exposure of lanes 1 and 2. (B) The remaining half of the sample was subjected to SDS-PAGE and immunoblot analysis with anti-HA to confirm the immunoprecipitation of HA-Snf1 and HA-Snf1K84R. We could not reproducibly detect LexA-Mig1 by immunoblot analysis of the immunoprecipitates. (C) Input proteins (10 μg) were analyzed by immunoblot analysis to verify the expression of LexA-Mig1. The phosphorylated LexA-Mig1 product detected in panel A corresponds to the lower band in this panel. Numbers on the right of each panel are molecular masses in kilodaltons.