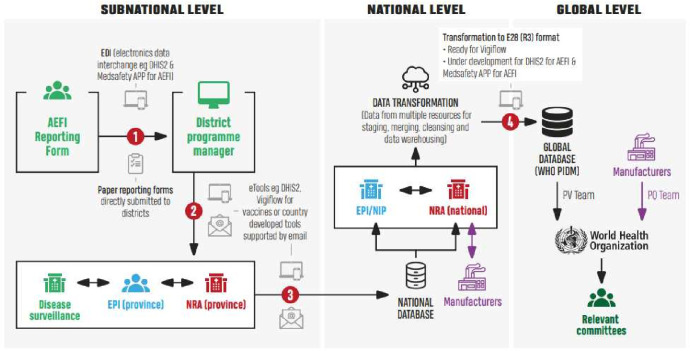
Figure 2.
Schematic representation of the structure for data sharing at the subnational, national and global levels as provided in the WHO COVID-19 vaccine safety surveillance manual. AEFI, adverse event following immunisation; EPI, expanded programmes on immunisation; NIP, national immunisation programme; NRA, national regulatory authority; PV, pharmacovigilance; PQ, prequalification; WHO PIDM, WHO Program for International Drug Monitoring.