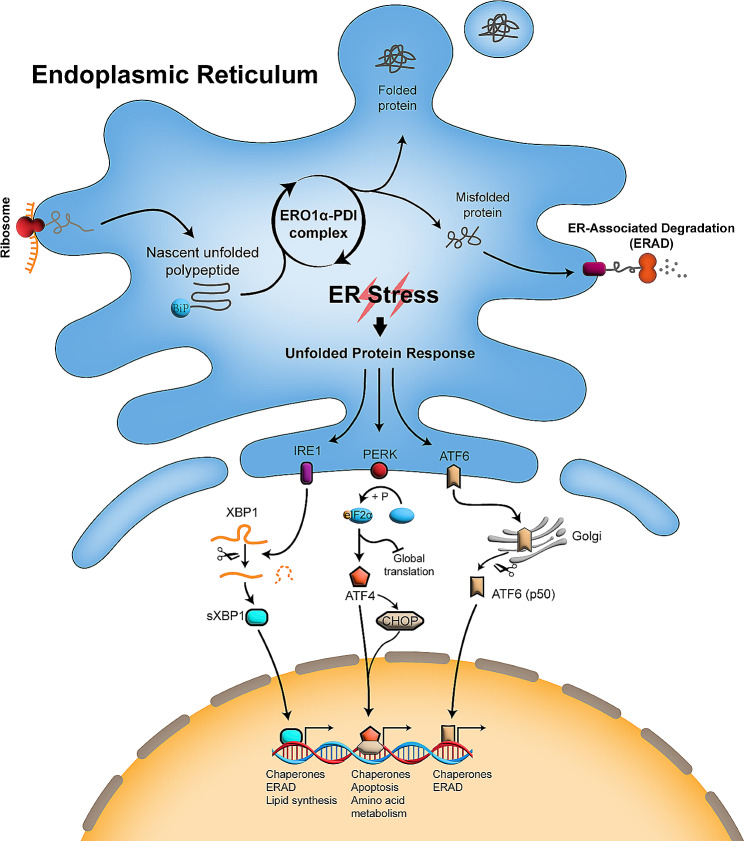
Fig. 1.
The oxidative protein folding in the ER and the unfolded protein response. Nascent polypeptides from ribosomes are oxidatively folded by the ERO1α-PDI complex. Dysfunctional ERO1α-PDI complex results in accumulation of unfolded and misfolded protein, which then arouses the UPR and sends signals to retard translation and facilitate processing and degradation of protein. During the UPR activation, ATF6 is transported to the Golgi apparatus, where it is processed to its activating form ATF6 (p50) and then locates to the nucleus for ERAD-related gene transcription. Activated PERK phosphorylates eIF2α, leading to global translation inhibition but selectively inducing ATF4. AFT4 then enters into the nuclear and activates gene transcription. Of note, facing the overwhelming ER stress, ATF4 can also activate CHOP, a transcription factor then inducing apoptosis through the caspase pathway. Activation of IRE1 induces the splicing of XBP1 and its activating form sXBP1 then goes into the nucleus and initiates the ERAD-related gene transcription. ERO1α, endoplasmic reticulum oxidoreductase 1 alpha; PDI, protein disulfide isomerase; BiP, binding immunoglobulin protein; ERAD, endoplasmic reticulum (ER)-associated degradation; UPR, unfolded protein response; IRE1, inositol requiring enzyme 1; PERK, protein kinase (PKR)-like ER kinase; ATF6, activating transcription factor 6; eIF2α, eukaryotic initiation factor 2α; XBP1, X-box binding protein 1; sXBP1, spliced XBP1; CHOP, C/EBP homologous protein