Abstract
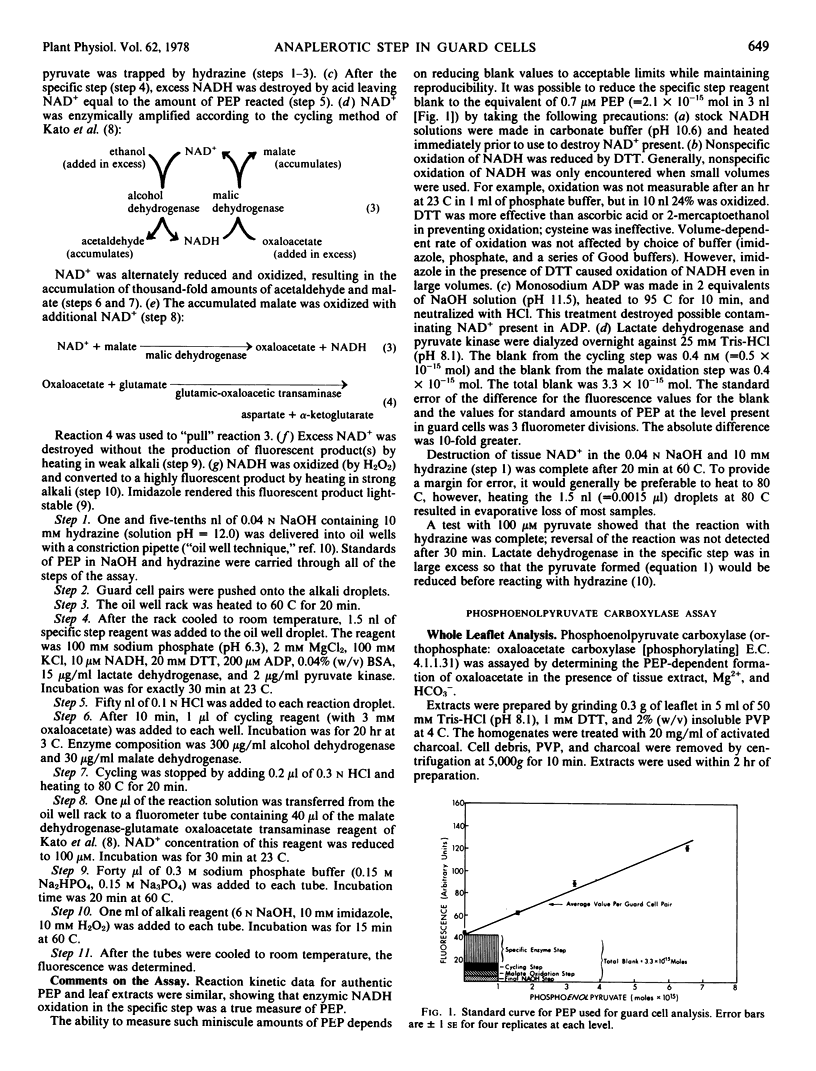
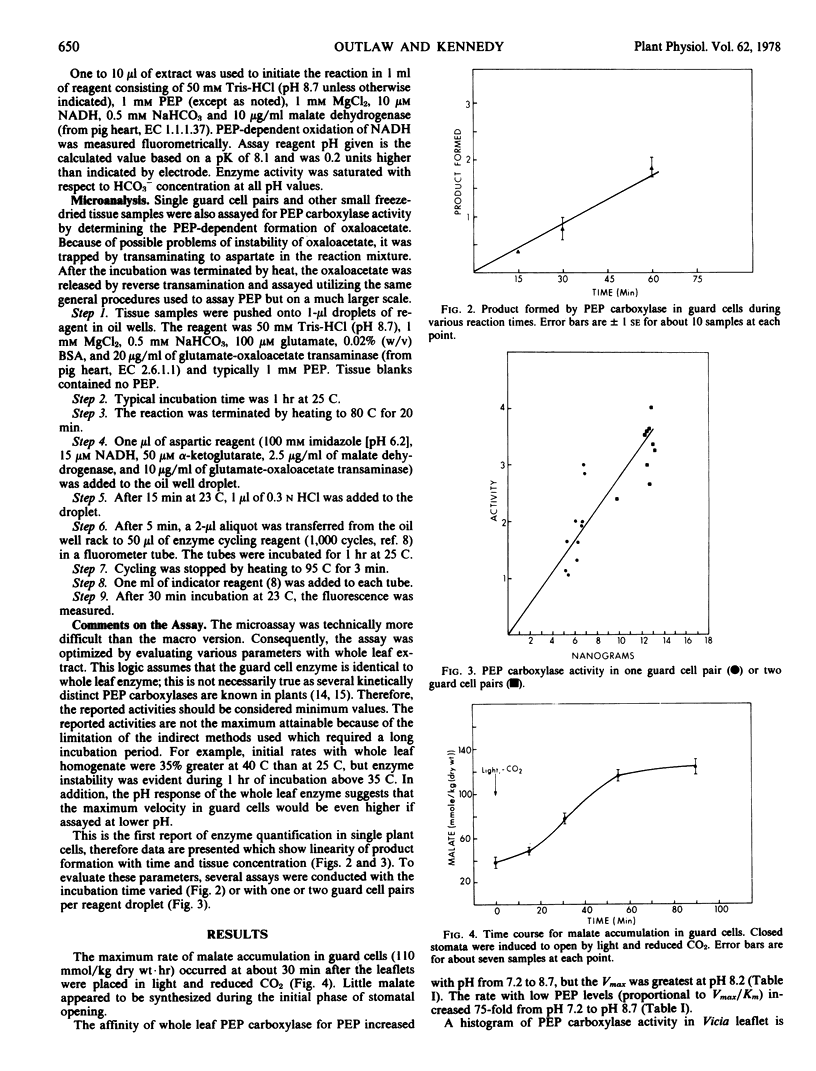
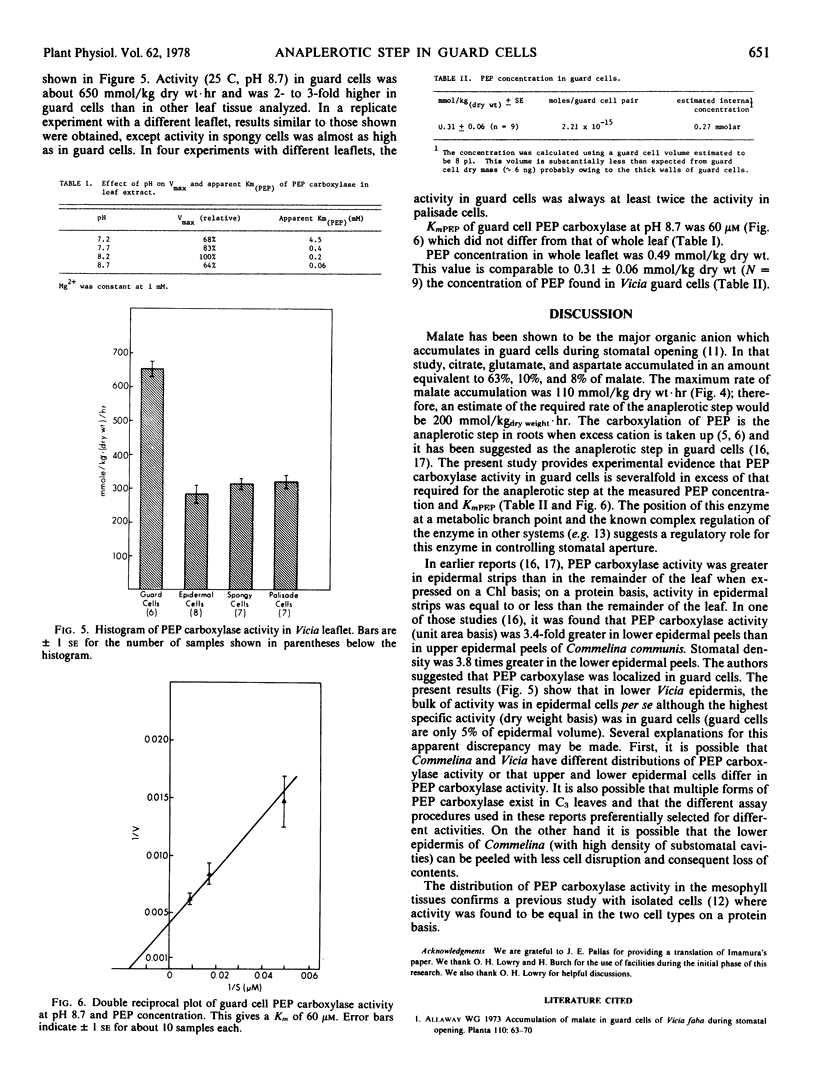
From the maximum rate of malate accumulation in Vicia faba L. guard cells during stomatal opening the maximum rate of organic anion synthesis is calculated to be 200 millimoles per kilogram dry weight per hour. A minimum estimate for the phosphoenolpyruvate (PEP) carboxylase-catalyzed reaction in guard cells is 650 millimoles per kilogram dry weight per hour which is significantly higher than in any other leaf tissue. The apparent Kmpep of the guard cell enzyme is 60 μm at pH 8.7, but is probably higher at lower pH. The concentration of PEP in guard cells was 270μm (=2.2 × 10−15 moles/guard cell pair) during anion synthesis. These results support the possibility that the carboxylation of PEP is the anaplerotic step in guard cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jacoby B., Laties G. G. Bicarbonate Fixation and Malate Compartmentation in Relation to Salt-induced Stoichiometric Synthesis of Organic Acid. Plant Physiol. 1971 Apr;47(4):525–531. doi: 10.1104/pp.47.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Berger S. J., Carter J. A., Lowry O. H. An enzymatic cycling method for nicotinamide-adenine dinucleotide with malic and alcohol dehydrogenases. Anal Biochem. 1973 May;53(1):86–97. doi: 10.1016/0003-2697(73)90409-0. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Carter J. G. Stabilizing the alkali-generated fluorescent derivatives of NAD and NADP. Anal Biochem. 1974 Jun;59(2):639–642. doi: 10.1016/0003-2697(74)90319-4. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Lowry O. H. Organic acid and potassium accumulation in guard cells during stomatal opening. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4434–4438. doi: 10.1073/pnas.74.10.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Schmuck C. L., Tolbert N. E. Photosynthetic Carbon Metabolism in the Palisade Parenchyma and Spongy Parenchyma of Vicia faba L. Plant Physiol. 1976 Aug;58(2):186–189. doi: 10.1104/pp.58.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. E. Escherichia coli phosphoenolpyruvate carboxylase: studies on the mechanism of multiple allosteric interactions. Arch Biochem Biophys. 1977 Oct;183(2):538–552. doi: 10.1016/0003-9861(77)90389-7. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Multiple forms of plant phosphoenolpyruvate carboxylase associated with different metabolic pathways. Plant Physiol. 1973 Mar;51(3):448–453. doi: 10.1104/pp.51.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer C. M., Pallas J. E., Black C. C. Carbon dioxide metabolism in leaf epidermal tissue. Plant Physiol. 1973 Nov;52(5):448–452. doi: 10.1104/pp.52.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer C., Kanai R., Pallas J. E., Jr, Black C. C., Jr Detection of high levels of phosphoenolpyruvate carboxylase in leaf epidermal tissue and its significance in stomatal movements. Life Sci II. 1973 Feb 22;12(4):155–159. [PubMed] [Google Scholar]


