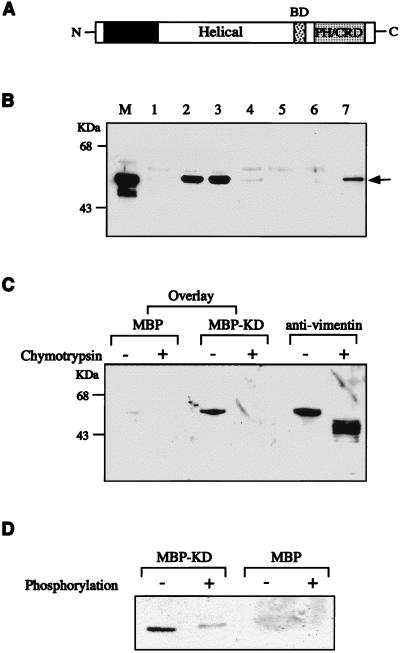
FIG. 3.
Association of ROKα and vimentin in vitro. (A) A schematic diagram of ROKα. BD, p21 binding domain; PH/CRD, PH/cysteine-rich domain. (B) Purified vimentin from Swiss 3T3 cells was incubated with MBP or GST fusion proteins containing different domains of ROKα. The lanes include MBP (lane 1), MBP-ROKα kinase domain (lane 2), MBP-ROKα KD (lane 3), MBP-ROKα helical coiled-coil domain (lane 4), GST (lane 5), GST-PH/cysteine-rich domain (lane 6), and GST-full-length ROKα (lane 7). An aliquot of the original mixture was loaded in lane M. The proteins were precipitated with amylose-Sepharose beads (lanes 1 to 4) or glutathione-Sepharose beads (lanes 5 to 7), separated by SDS-PAGE, analyzed by Western blotting, and monitored by ECL to detect the bound vimentin (arrow) with antivimentin antibody. (C) Binding of ROKα to the head domain of vimentin. A filter overlay was carried out on a nitrocellulose filter containing purified vimentin digested in the presence or absence of chymotrypsin. The filter was first overlaid with 1 μg of MBP or MBP-KD (kinase-dead domain of ROKα) (amino acids 1 to 468) per ml and then probed with rabbit anti-MBP and horseradish peroxidase-conjugated anti-rabbit IgG antibodies. The filter was reprobed with antivimentin antibody to confirm the location of the proteins. (D) The ROKα KD has reduced affinity for phosphorylated vimentin. GST-vimentin head domain (1 μg) purified from E. coli was phosphorylated in the presence or absence of ATP, subjected to the same overlay treatment with either MBP or MBP-KD as probe as described for panel C, and immunostained with anti-MBP antibody. Film images were digitized with a Microtek scanner, cropped, converted to gray scale with Adobe Photoshop 4.0, and printed with an Epson Stylus Color 800 inkjet printer.