Abstract
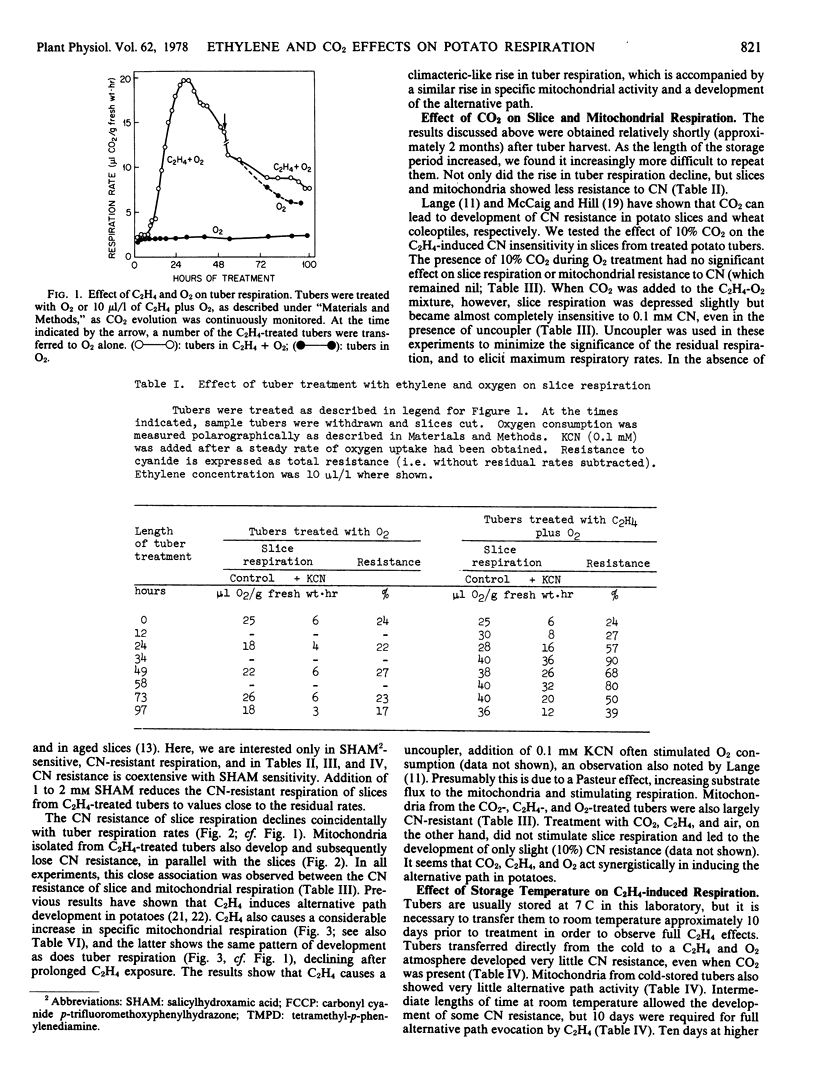
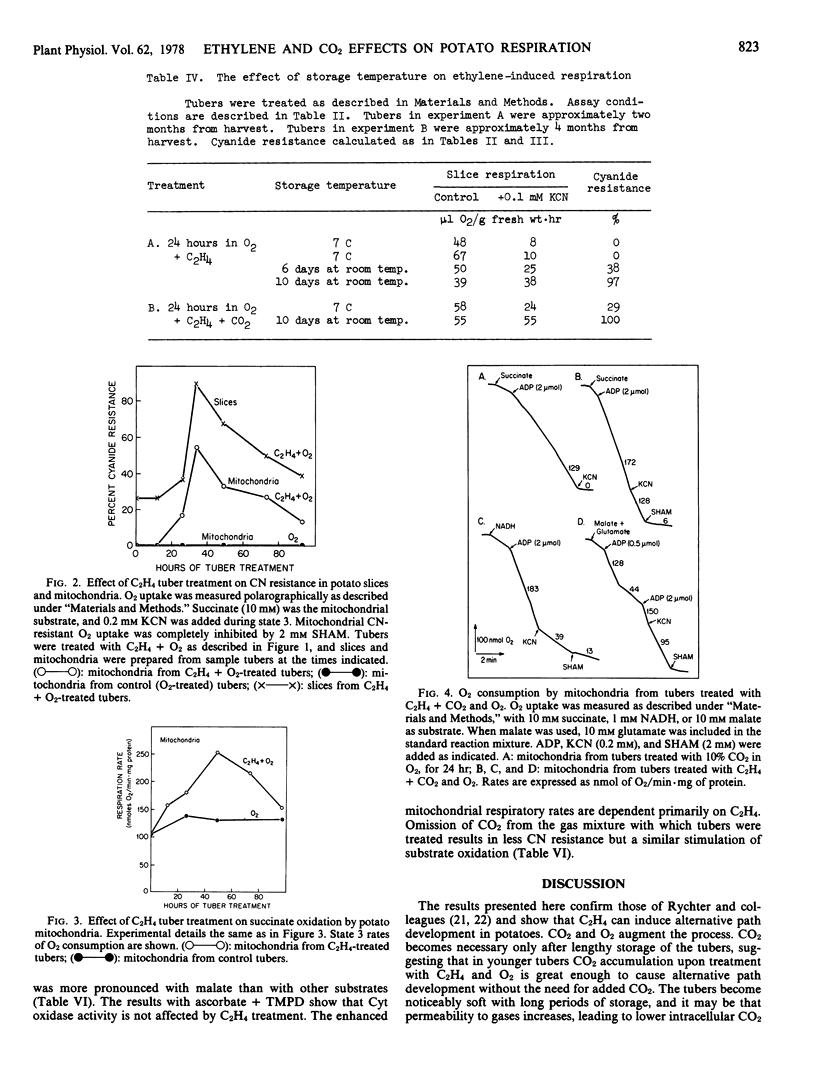
The respiration of potato tubers (Solanum tuberosum var. Russet Burbank) which have been kept at room temperature for 10 days is stimulated upon subsequent treatment with C2H4 (10 microliters per liter) and O2. The respiratory rise reaches a peak in 24 to 30 hours and thereafter declines. Coincident with the rise in tuber respiration is an increase in the respiratory rates of fresh slices and isolated mitochondria. Slices and mitochondria from C2H4- and O2-treated tubers also display substantial resistance to CN, and the resistant respiration is inhibited by hydroxamates.
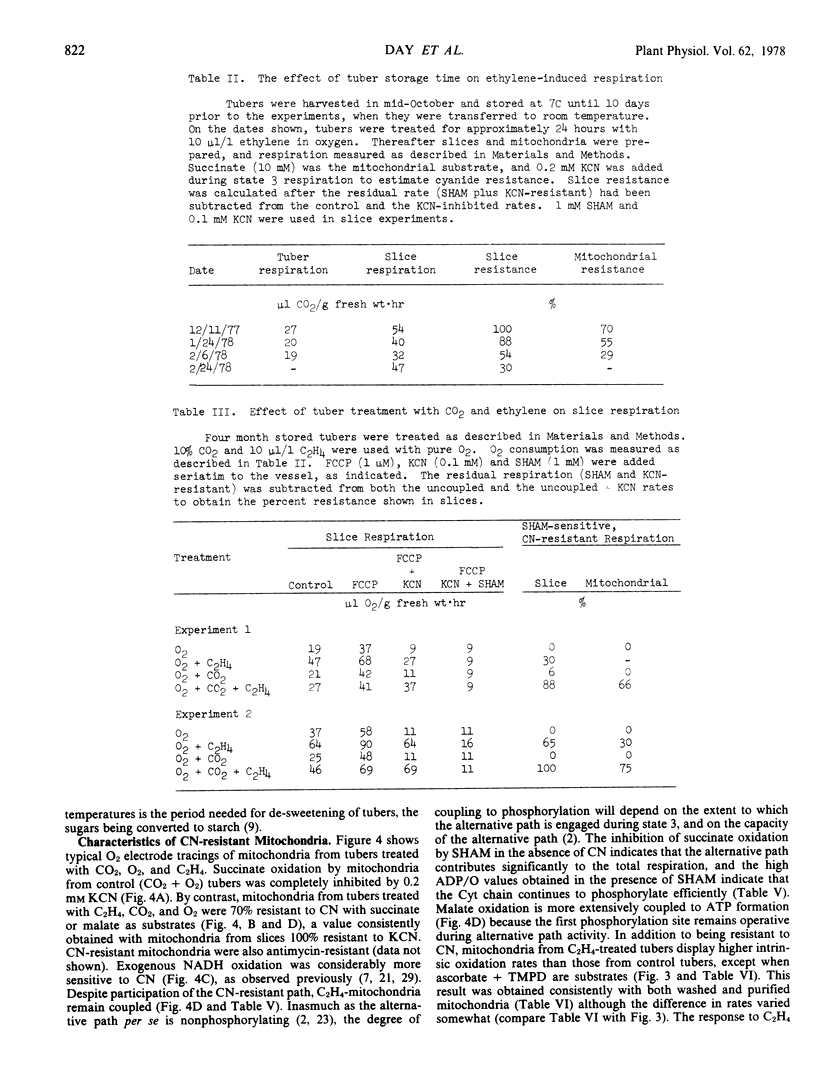
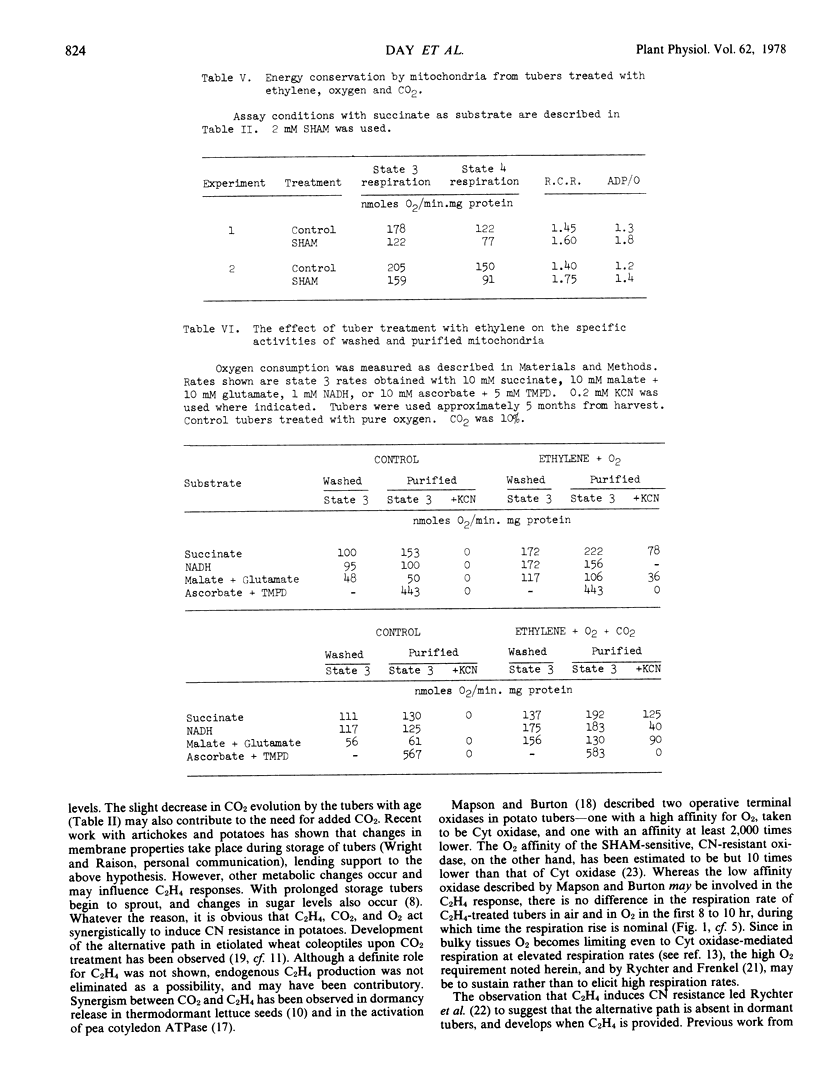
The longer the tubers are stored after harvest, the less effective is C2H4 in causing CN resistance in slices and mitochondria from treated tubers. Addition of 10% CO2 to the C2H4-O2 mixture, however, causes extensive CN resistance to develop, even in slices and mitochondria from old tubers. The results show that C2H4, O2, and CO2 act synergistically to induce alternative path development in potatoes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973 May 25;248(10):3446–3450. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Chin C. K., Frenkel C. Upsurge in respiration and peroxide formation in potato tubers as influenced by ethylene, propylene, and cyanide. Plant Physiol. 1977 Mar;59(3):515–518. doi: 10.1104/pp.59.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizengremel P., Lance C. Control of Changes in Mitochondrial Activities during Aging of Potato Slices. Plant Physiol. 1976 Aug;58(2):147–151. doi: 10.1104/pp.58.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys R. D., Smith O. E., Kumamoto J., Lyon J. L. Effect of Gibberellic Acid, Kinetin, and Ethylene plus Carbon Dioxide on the Thermodormancy of Lettuce Seed (Lactuca sativa L. cv. Mesa 659). Plant Physiol. 1975 Dec;56(6):826–829. doi: 10.1104/pp.56.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laties G. G., Hoelle C. Malonate and Cyanide Insensitivity in Relation to Respiratory Compensation in Potato Slices. Plant Physiol. 1965 Jul;40(4):757–764. doi: 10.1104/pp.40.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G. The potentiating effect of adenosine diphosphate in the uncoupling of oxidative phosphorylation in potato mitochondria. Biochemistry. 1973 Aug 14;12(17):3350–3355. doi: 10.1021/bi00741a032. [DOI] [PubMed] [Google Scholar]
- MAPSON L. W., BURTON W. G. The terminal oxidases of the potato tuber. Biochem J. 1962 Jan;82:19–25. doi: 10.1042/bj0820019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S. S., Spencer M. Effects of ethylene, carbon dioxide, and ethylene - carbon dioxide mixtures on the activities of "membrane-containing" and "highly purified" preparations of adenosine triphosphatase from pea-cotyledon mitochondria. Can J Biochem. 1974 Dec;52(12):1091–1096. doi: 10.1139/o74-153. [DOI] [PubMed] [Google Scholar]
- Reid M. S., Pratt H. K. Effects of ethylene on potato tuber respiration. Plant Physiol. 1972 Feb;49(2):252–255. doi: 10.1104/pp.49.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychter A., Janes H. W., Frenkel C. Cyanide-resistant Respiration in Freshly Cut Potato Slices. Plant Physiol. 1978 Apr;61(4):667–668. doi: 10.1104/pp.61.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Induction of ethylene of cyanide-resistant respiration. Biochem Biophys Res Commun. 1976 May 17;70(2):663–671. doi: 10.1016/0006-291x(76)91098-6. [DOI] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Similarities between the Actions of Ethylene and Cyanide in Initiating the Climacteric and Ripening of Avocados. Plant Physiol. 1974 Oct;54(4):506–511. doi: 10.1104/pp.54.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. The mechanism of ethylene and cyanide action in triggering the rise in respiration in potato tubers. Plant Physiol. 1975 Jan;55(1):73–78. doi: 10.1104/pp.55.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Antimycin-insensitive Cytochrome-mediated Respiration in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):238–242. doi: 10.1104/pp.62.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson P. F., Moreland D. E. Cyanide-resistant Respiration of Sweet Potato Mitochondria. Plant Physiol. 1975 Feb;55(2):365–369. doi: 10.1104/pp.55.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring A. J., Laties G. G. Inhibition of the Development of Induced Respiration and Cyanide-insensitive Respiration in Potato Tuber Slices by Cerulenin and Dimethylaminoethanol. Plant Physiol. 1977 Jul;60(1):11–16. doi: 10.1104/pp.60.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]


