Fig. 1 |. Significance, challenges and strategy for accessing multi-substituted BCPs.
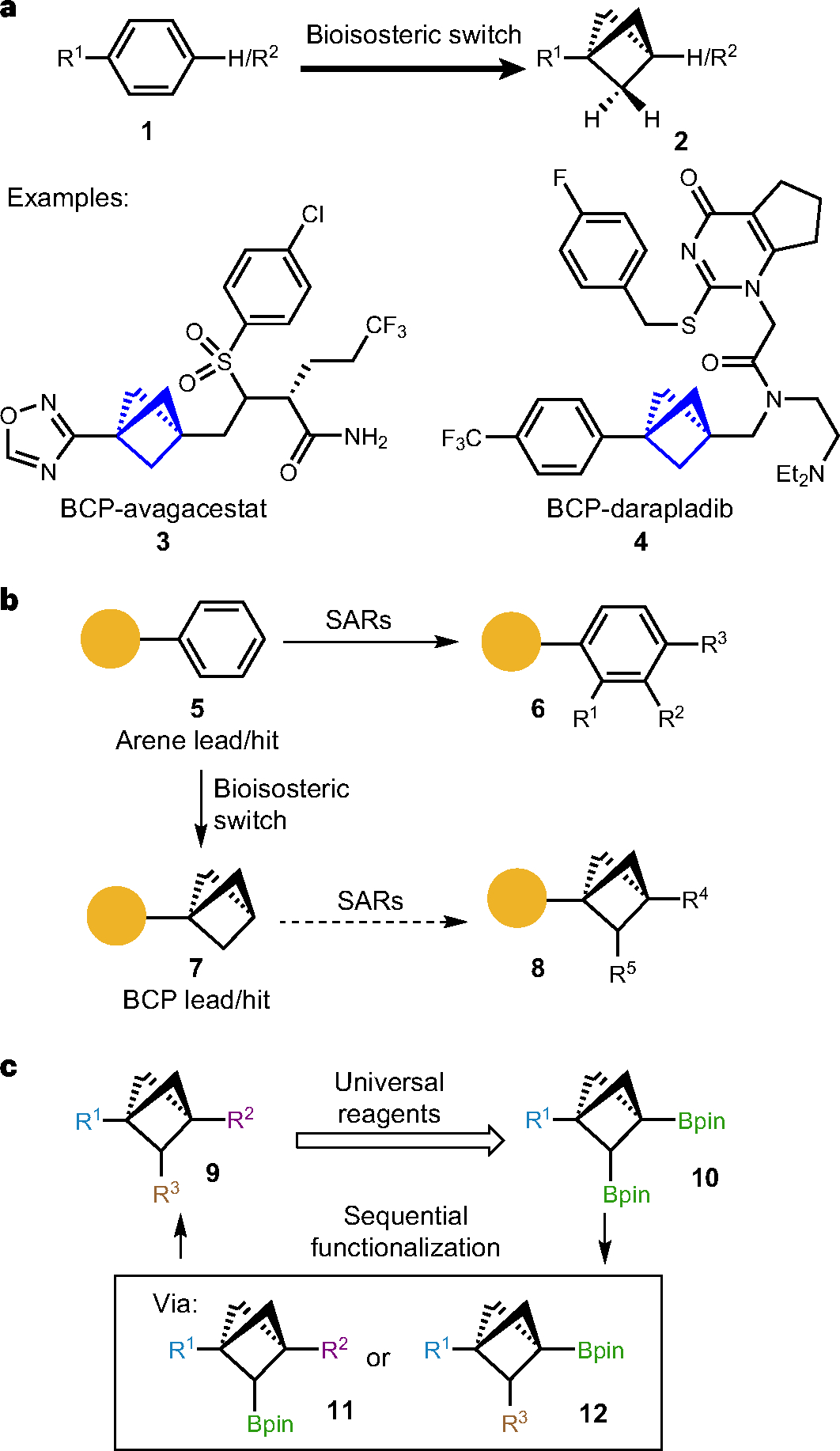
a, Significance of bicyclo[1.1.1]pentanes as a 3D bioisostere for arenes in medicinal chemistry. Bicyclo[1.1.1]pentanes has been applied as benzene 3D-surrogates in drug discovery owing to their metabolic stability from their high BDE and kinetically inhibited HAT process. Several BCP derivatives of drug molecules such as BCP-avagacestat and BCP-darapladib have demonstrated their potential to improve physicochemical, pharmacological (ADME) and toxicological (safety) properties. b, Synthetic challenges in SAR studies with a BCP core structure. Owing to well-established C(sp2)–C(sp2) cross-coupling reactions and abundant available building blocks, it is synthetically accessible to obtain diverse derivatives of arene lead compounds for further SAR studies; however, derivatization of the C(sp3)-rich bioisostere BCPs has been synthetically challenging due to non-programmable synthetic route, requirement of de novo synthesis and inaccessible chemical space. c, Programmable and sequential functionalization of bridge-substituted BCPs. To access multisubstituted BCPs (9), a sequential functionalization strategy from BCP bis-boronate (10) was proposed taking advantage of different reactivity of two boronates. Diversification of R1, chemoselectivity of two boronates and reactivity of boronic esters are main challenges in this strategy.
