Fig. 4 |. Syntheses of structurally divergent BCP compounds.
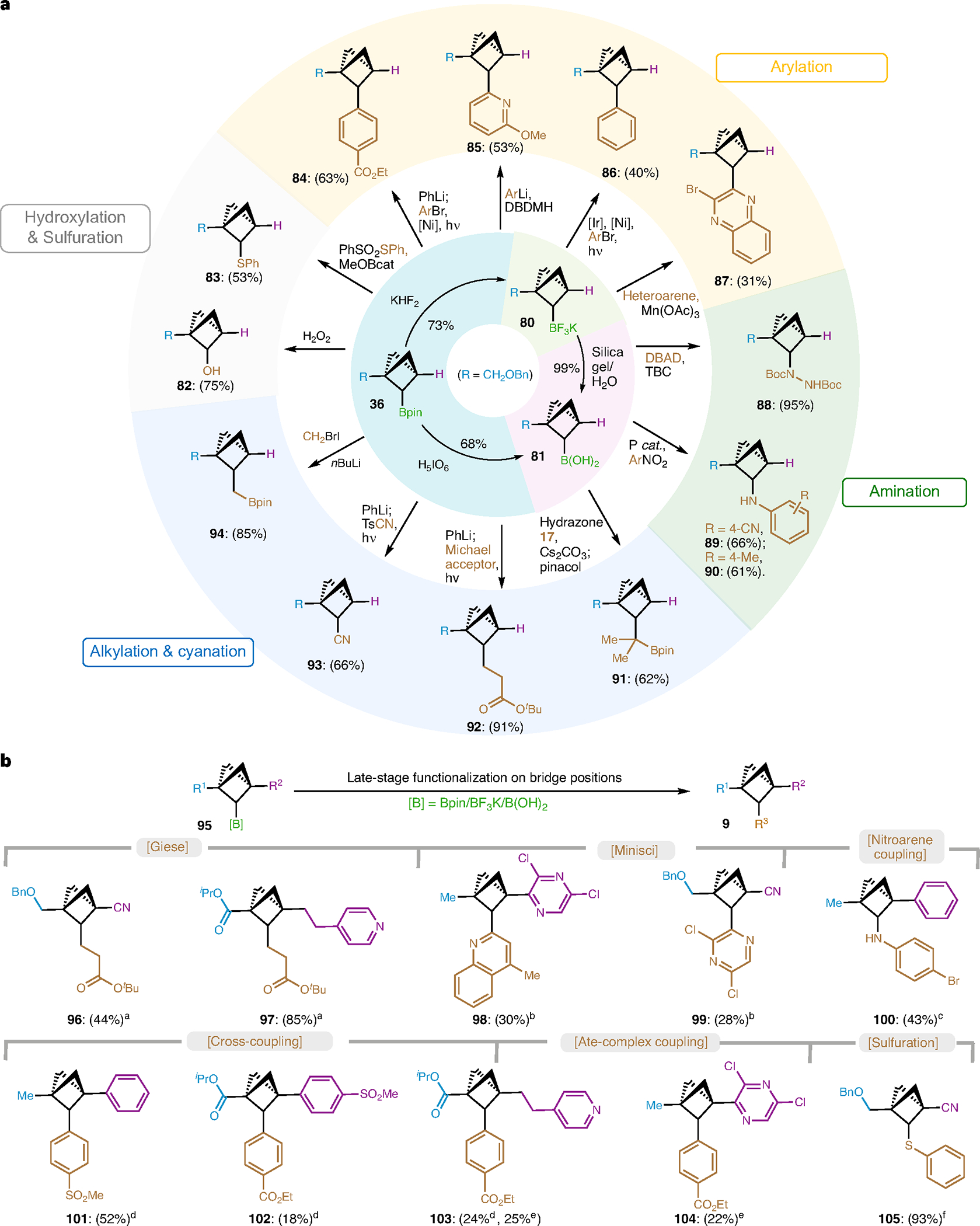
a, Functionalization of C2-Bpin as a versatile building block towards C1, C2-disubstituted BCPs. b, Late-stage functionalization to access C1, C2, C3-trisubstituted BCPs. Reaction conditions: aBCP C2-Bpin (1.0 equiv.), PhLi (1.2 equiv.) THF (0.2 M), −78 °C to r.t., 1 h; then 4-CzlPN (5 mol%), tert-butyl acrylate (2.0 equiv.), THF/MeCN (0.1 M), blue LEDs, 15 h. bBCP C2-BF3K (1.0 equiv.), heteroarene (3.0 equiv.), Mn(OAc)3 (2.5 equiv.), TFA (2.5 equiv.), AcOH/H2O (1:1, 0.1 M), 50 °C, 18 h. cBCP C2-B(OH)2 (1.0 equiv.), ArNO2 (1.0 equiv.), 1,2,2,3,4,4-hexamethyl-phosphetane 1-oxide (15 mol%), PhSiH3 (2.0 equiv.), m-xylene (0.5 M), 120 °C, 8 h. dBCP C2-BF3K (1.0 equiv.), [Ir] (5 mol%), Ni(dtbbpy) Cl2 (20 mol%), ArBr (5.0 equiv.), Cs2CO3 (6.0 equiv.), dioxane or THF (0.1 M), blue LEDs, 24 h. eBCP C2-Bpin (1.0 equiv.), PhLi (1.2 equiv.) THF (0.2 M), −78 °C to r.t., 1 h; then 4-CzlPN (5 mol%), Ni(dtbbpy)Cl2 (10 mol%), ArBr (3.0 equiv.), THF/DMA (0.1 M), blue LEDs, 15 h. fBCP C2-Bpin (1.0 equiv.), PhSO2SPh (4.0 equiv.), MeOBcat (1.0 equiv.), TBC (0.3 equiv.), toluene (0.2 M), 80 °C, 24 h. See the ‘Experimental procedures and characterization data of substrates’ section in Supplementary Information for details.
