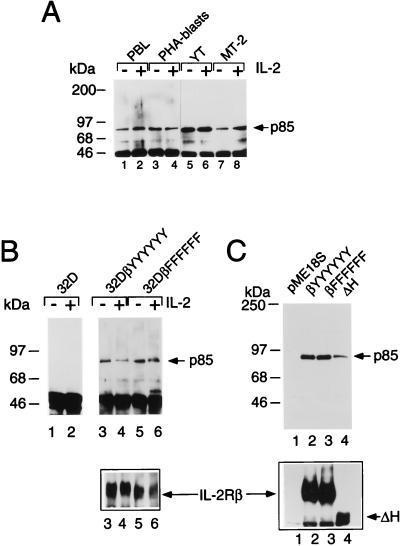
FIG. 1.
Tyrosine residues of IL-2Rβ are not required for its association with p85. (A) Constitutive association of IL-2Rβ and p85. Freshly isolated PBL (lanes 1 and 2), PHA blasts (lanes 3 and 4), YT cells (lanes 5 and 6), or MT-2 cells (lanes 7 and 8) were starved for ≥4 h in RPMI 1640 medium containing 1% FBS and were not stimulated (lanes 1, 3, 5, and 7) or were stimulated with 2 nM IL-2 (lanes 2, 4, 6, and 8) for 10 min; cell lysates were immunoprecipitated with hMikβ1 and immunoblotted with anti-p85. (B) Coprecipitation of p85 with IL-2Rβ in 32D cells expressing wild-type IL-2Rβ (lanes 3 and 4) or a mutated form of IL-2Rβ in which all six tyrosines were mutated to phenylalanines (lanes 5 and 6). Cells were starved of growth factor for 4 h, not stimulated or stimulated with 2 nM IL-2 for 10 min, washed, and lysed prior to immunoprecipitation and Western blotting. (C) Coprecipitation of p85 with IL-2Rβ constructs. 293 T+ cells were cotransfected with plasmids expressing p85, Jak1, and either the empty vector (pME18S; lane 1) or pME18S driving expression of wild-type IL-2Rβ (βYYYYYY; lane 2), IL-2Rβ in which all tyrosines are mutated (βFFFFFF; lane 3), or an IL-2Rβ construct lacking residues 380 to 525 (ΔH mutant; lane 4). Cells were harvested 36 to 48 h posttransfection. Note that the decrease in apparent p85 coprecipitation (lane 4, top) corresponded to the decreased expression of IL-2Rβ ΔH (lane 4, bottom). Immunoprecipitation and Western blotting for panels B and C were performed as for panel A. The lower portions of panels B and C represent Western blots with goat antiserum to human IL-2Rβ (R & D Systems), as controls for expression.