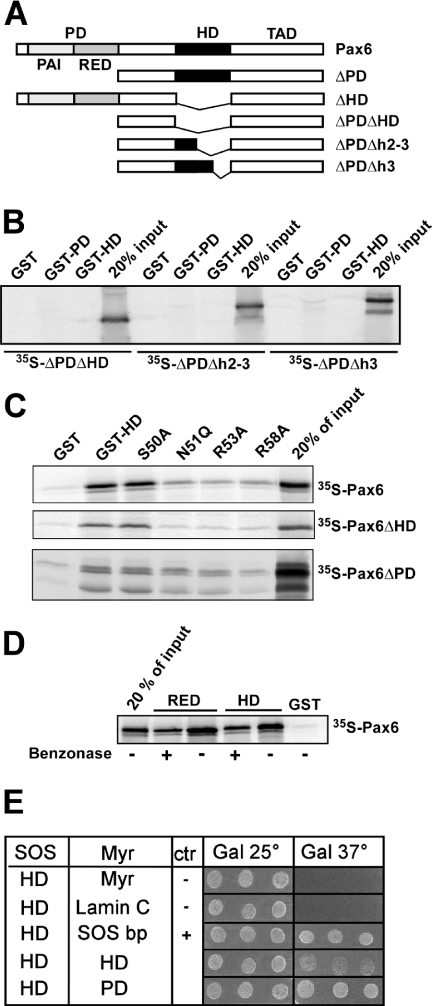
Figure 1.
The recognition helix of the homeodomain of Pax6 is important for interaction with both the PD and the HD. (A) Pax6 constructs used for in vitro translation and GST pull-downs. (B) GST pull-down assays with Pax6 HD and PD fused to GST and immobilized on glutathione–agarose beads and Pax6ΔPDΔHD, Pax6ΔPDΔh2–3 or Pax6ΔPDΔh3 produced by in vitro transcription and translation in the presence of [35S]methionine. An aliquot of 10 μl of the in vitro translation reactions was preincubated with GST immobilized on glutathione–agarose beads before incubation with the GST fusion proteins. The GST beads, GST-Pax6 HD beads and GST-Pax6 PD beads were washed several times before they were boiled in SDS loading buffer and run on a 10% SDS–polyacrylamide gel. An aliquot of 2 μl of the in vitro translated proteins was run on the same gel to visualize the signal from 20% of the input. (C) Point mutations in helix 3 of the homeodomain strongly reduce the ability of Pax6 HD to interact with the PD and the wild-type HD. The N51Q, R53A and R58A, but not S50A, mutants impede the HD–PD and HD–HD interactions. GST pull-down assays were performed with recombinant GST fusions of wild type or mutants of Pax6ΔHD against in vitro translated, [35S]methionine-labeled Pax6, Pax6ΔHD or Pax6ΔPD. (D) The interactions between full-length Pax6 and the RED subdomain and between full-length Pax6 and the HD are independent of DNA. GST pull-down assays were done with Pax6 HD and RED fused to GST as in (C). Where indicated, the pull-down experiments were performed in the presence of 500 U benzonase to degrade both DNA and RNA. The results shown are representative of three independent experiments. (E) The PD–HD and HD–HD interactions of Pax6 are also observed in the yeast-based SOS recruitment interaction system. The temperature sensitive yeast strain S.cerevisiae cdc25-2 MATa was co-transformed either with pSOS-zfPax6-HDwt and empty pMYR or pMYR-LaminC as negative controls, pMYR-SOS binding protein as a positive control, pMYR-zfPax6-HDwt, or with pMYR-zfPax6-PDwt. Three independent colonies generated from each co-transformation were replica plated onto galactose plates and grown in parallel at 25 and 37°C for 6 days. The results shown are representative of three independent experiments.