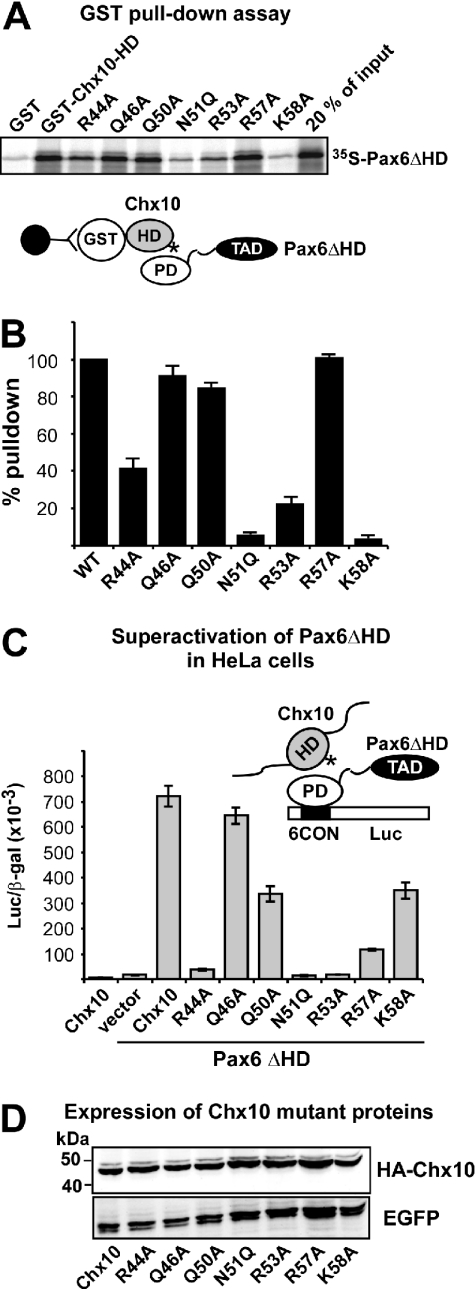
Figure 7.
Mutation of basic amino acids in helix 3 of the Chx10 homeodomain leads to reduced interaction with and superactivation by Pax6ΔHD. (A) GST pull-down assays with Chx10 HD wild type and mutants fused to GST and immobilized on glutathione–agarose beads and Pax6ΔHD protein produced by in vitro transcription and translation in the presence of [35S]methionine. (B) Quantitative representation of the interaction data determined as described in the legend to Figure 3. (C) Effects of mutations in the recognition helix of the HD of Chx10 on superactivation of Pax6ΔHD-mediated transactivation from paired domain-binding sites. HeLa cells were co-transfected with 0.5 μg Pax6ΔHD, 0.5 μg pP6CON-LUC and 5 ng pCMV-βgal together with either 0.25 μg pcDNA3-HA vector, HAChx10 or HA-Chx10 mutants. HA-Chx10 co-transfected with the empty Pax6ΔHD control vector shows that Chx10 alone does not activate the P6CON LUC reporter. The data in (B) and (C) represent the mean of three independent experiments. (D) Western blot showing similar expression levels of wild type and all helix 3 mutants of Chx10 following transfection of HeLa cells. EGFP served as transfection control.