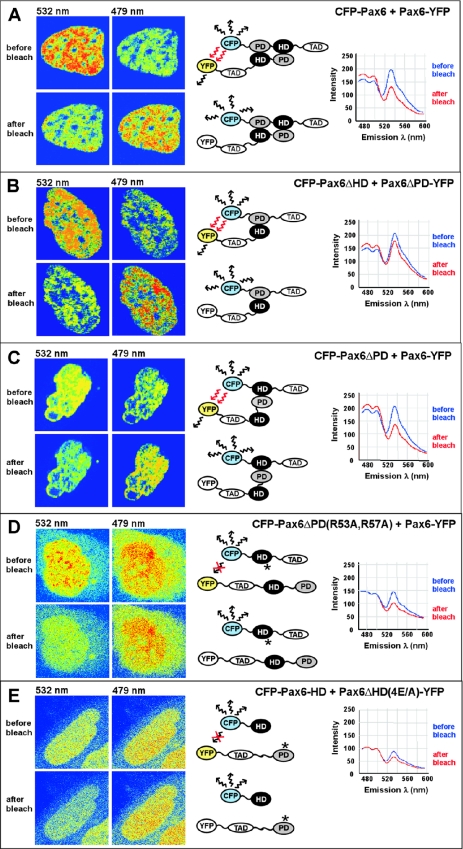
Figure 8.
The paired domain and homeodomain of Pax6 interact directly with each other, as analyzed by using FRET. The CFP- and YFP-tagged Pax6 proteins indicated in (A–E) were co-expressed in human HeLa cells. The cell images are visualized in pseudocolors before and after acceptor photobleaching to highlight changes in fluorescence intensity. Images of CFP and YFP fluorescence were obtained using the 458 nm laser line. The YFP acceptor was bleached in the whole nucleus using the 514 nm laser line. FRET was detected as decreased YFP-emission at 532 nm and a corresponding increased CFP-emission at 479 nm in the bleached area. (A) Full-length Pax6 proteins homodimerize in the nucleus of living cells. (B) The paired domain of Pax6ΔHD interacts directly with the homeodomain of the paired-less isoform Pax6ΔPD. (C) FRET analysis demonstrates interactions between the homeodomain of Pax6ΔPD and the paired domain of the full-length Pax6 isoform. (D) Mutations of arginine residues in helix 3 of the homeodomain of Pax6 impair the interaction with the paired domain, as demonstrated by no visible FRET between the HD double mutant R53A/R57A of Pax6ΔPD and the PD of full-length Pax6. (E) No FRET was detected between CFP and YFP in cells expressing a quadruple mutation in the paired domain of Pax6ΔHD-E101A/E112A/E120A/E128A and the wild-type homeodomain of Pax6. Cells expressing approximately a 1:1 ratio of the CFP- and YFP-tagged proteins were used for FRET experiments.