Abstract
Background:
Family-based behavioral treatment (FBT) is an effective intensive health behavior and lifestyle treatment for obesity reduction in children and adolescents, but families have limited access. The purpose of this randomized, pragmatic, comparative effectiveness trial was to examine changes in child relative weight in a 12-month, enhanced standard of care (eSOC) intervention combined with FBT (eSOC+FBT) vs. eSOC alone.
Methods:
Children aged 6 to 15 years with obesity, and their primary caregiver, were recruited from primary care clinics. Families were randomized 1:1 to eSOC, a staged approach led by the primary care provider that gradually intensified dependent on a child’s response to care and aligns with the American Medical Association guidelines, or the eSOC+FBT arm, which included regular meetings with a health coach for healthy eating, physical activity, positive parenting strategies, and managing social and environmental cues. Both treatments align with the 2023 American Academy of Pediatrics clinical practice guidelines. Assessments occurred at baseline, midpoint (month 6), end-of-intervention (month 12), and follow-up (month 18). Primary outcome was change from baseline to 12 months in child percent overweight (percentage above the median body mass index in the general US population normalized for age and sex). Secondary outcomes were parent weight, child psychosocial factors, heterogeneity of treatment effects, and cardiometabolic risk factors. Exploratory outcomes assessed reach, effectiveness, adoption, implementation, and maintenance.
Conclusion:
This pragmatic trial will generate evidence for the comparative effectiveness of implementing two guidelines-based approaches in primary care for obesity reduction in children and adolescents.
Trial registration:
ClinicalTrials.gov Identifier: NCT03843424
Keywords: Health coaches, intensive lifestyle intervention, weight loss, primary care
Childhood obesity remains an urgent public health concern, with one in five U.S. children between the ages of 2 and 19 having obesity.1,2 Youth with obesity are five times more likely to have obesity as adults compared to peers with healthy weight.3 Pediatric obesity contributes to cardiometabolic risk,4 poor sleep,5 type 2 diabetes,6 decreased quality of life,7 and depression.8 These negative health outcomes are even more prevalent among the uninsured and underinsured9 and among children who are historically marginalized,10 further exacerbating health disparities.
The 2023 American Academy of Pediatrics (AAP) Clinical Practice Guideline (CPG) for the Evaluation and Treatment of Children and Adolescents with Obesity recommended intensive health behavior and lifestyle treatment (IHBLT) programs as an effective approach that should be offered to all children and adolescents with obesity.11 IHBLT delivers at least 26 hours of family-based counseling over a 3- to 12-month period for children 6 years and older with overweight and obesity, a recommendation also consistent with the 2017 U.S. Preventive Services Task Force (USPSTF) guidelines.12 Family-based behavioral treatment (FBT) meets these recommendations and has been found to effectively reduce child percent overweight by up to almost 20%.13 FBT programs are comprehensive and include behavioral modification, positive parenting practices, environmental modification, and a focus on nutrition and physical activity counseling.14
The 2023 AAP CPG also endorsed the role of the primary care provider (PCP) to deliver counseling on nutrition and physical activity (referred to as “enhanced standard of care” [eSOC] for this study). In recognition that many PCPs do not have access to IHBLT, the AAP CPG recommends that pediatricians and other pediatric healthcare providers increase the intensity of weight management support by connecting families with resources to support nutrition and physical activity based on the availability of local dietitians and community programs. This approach aligns with the prior 2007 American Medical Association (AMA) staged approach that begins with prevention counseling by the PCP and gradually escalates as indicated to structured meal and physical activity plans, and then finally to IHBLT programs, medication, and surgery when available and when prior efforts fail to produce weight loss.15
There remain gaps in the dissemination and implementation of FBT programs in primary care settings. The first gap is the need for primary-care feasible interventions.16 While primary care locations are promising for the dissemination of feasible and efficacious treatments,17 more information is needed on their consistency with national recommendations for pediatric weight management. Further, telehealth is a growing option but remains understudied as a mode to deliver eSOC or IHBLT. The second gap is in understanding FBT outcomes among racially and ethnically diverse samples. Despite differences in prevalence of obesity among White, Hispanic, and Black children,10 less research is available on the treatment effects of FBT among these groups.18 Some FBT trials have found no differences in weight outcomes, following treatment, for Hispanic versus non-Hispanic participants,19 but less is known about differences among White versus Black children.20 A third gap is potential differences in treatment effects between girls and boys.21 Research between adult men and women suggest differences in reductions in weight and adiposity,22 treatment adherence23 and participation.24 Less is known among pediatric samples about these important sex-specific differences. Finally, FBT programs delivered in specialty care or academic research settings have benefited the caregiver,25,26 but FBT delivered in a pediatric primary-care setting for adult and child weight loss remains under-evaluated though initial results are promising.18 The current study aimed to address these important gaps in the evidence.16
The Treatment Efforts Addressing Child Weight Management by Unifying Patients, Parents, and Providers (TEAM UP) study was a randomized, pragmatic, comparative effectiveness trial that examined changes in child relative weight in a 12-month, eSOC with FBT (eSOC+FBT) vs. eSOC alone, both delivered in primary care. It was hypothesized that children and their parents/caregivers who received eSOC+FBT would have greater reductions in percent overweight compared to those who received eSOC alone. Secondary aims of the study included: 1) examine if children who receive the eSOC+FBT intervention will improve psychosocial factors relative to children who receive eSOC alone; 2) examine the heterogeneity of treatment effects (HTE) across participant subgroups; and 3) examine improvements in standard clinical and laboratory assessments of cardiometabolic outcomes. An exploratory aim of the study was to conduct process evaluations to assess RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance)27 domains across participants, providers, and practices.
Methods
Study Design
Participants were randomly assigned to 12-months of eSOC alone (n=376 child-parent/caregiver dyads) or eSOC+FBT (n=354 dyads), with primary child/parent measurements obtained at 6 (midpoint), 12 (end), and 18 (follow-up) month intervals. All participating children and parents/caregivers received eSOC delivered by a PCP. To examine the feasibility of implementing IHBLT within primary care settings, coaches delivered FBT and provided on-going care coordination with the child’s PCP.
Partner and Family Engagement
A Family Advisory Board allowed the study team to engage with families (non-study participants) throughout the development of study materials, active intervention, and dissemination of results. Parent focus groups were conducted to ensure feasible and understandable treatment components.28 A board of research and clinician scientists, the Evidence-Based Advisory Board, advised on implementation of evidence-based practices. The Provider Advisory Board, composed of pediatricians, family medicine physicians, nurse practitioners, dietitians, and behavioral counselors, advised on eSOC and FBT provider training, patient recruitment, intervention implementation in the clinic setting, and continuity after the study ended. The Payer Advisory Board guided the dissemination plan by defining indicators related to study outcomes that support advocacy for reimbursement of obesity services. Members of the Advisory Boards are listed in the Acknowledgements.
Participant Recruitment and Screening
All study procedures were approved by the Washington University in St. Louis Institutional Review Board (IRB), which served as the single IRB. Study staff were certified to the same protocol and manual of procedures across all sites; certification included written assessments and direct observation by lead data collectors during assessment visits. Families were recruited from clinical practices in primarily urban and suburban sites including greater Baton Rouge and greater New Orleans, Louisiana; Rochester, New York; greater St. Louis area and Columbia, Missouri extending into rural areas of the state; and suburbs of St. Louis in Illinois. This was a convenience sample with a target to recruit 50% non-white families to ensure diversity. Specific efforts were made a special focus on recruiting Black families, Hispanic families, and families insured by Medicaid. For example, some clinical practices were enrolled because of their high proportion of minority and Medicaid insured patients, and photographs and videos used in recruiting materials included people of diverse racial and ethnic backgrounds. Because of the pragmatic nature of the trial, the trial used broad eligibility with minimal exclusion criteria (see Table 1). The term parent/caregiver refers to the targeted adult who regularly attended treatment with the participating child.
Table 1.
Eligibility criteria for the TEAM UP trial.
| Inclusion Criteria (Child and Parent/Caregiver) | Exclusion Criteria (Child) |
|---|---|
|
Child Inclusion Criteria: • BMI percentile ≥95th for age and sex • Aged 6–15 years • Comfortable speaking English language • Able to provide written or verbal (based on age and preference) informed assent • Willing to change eating behaviors, physical activity, and/or weight • Patient of a participating clinic • Able to participate in scheduled sessions Parent/Caregiver Inclusion Criteria: • Aged ≥ 18 years • Comfortable speaking and reading English language • Child resides with the participating parent/caregiver ≥50% of the time |
Child Exclusion Criteria • Families who plan to no longer have the child be a patient of any participating clinic during any point in the 18-month study period • Families for whom the PCP or site Principal Investigator (PI) thinks the study and/or intervention is clinically/medically inappropriate (e.g., more than mild developmental delay, or emotional or cognitive difficulties, if the PI/PCP believes these factors will interfere with study/intervention participation) • Families in whom the parent or child exhibits purging behavior and/or other significant eating disorder symptomatology • Children with chronic conditions or on medications that substantially impact or interfere with growth, appetite, weight, or physical activity participation |
Recruitment strategies included face-to-face recruitment where the PCP referred interested families to study staff; electronic medical records (EMR) queries to identify eligible families who were approved by the PCPs to be contacted; and general advertisements in practices/clinics, on provider websites and social media, and in targeted social media advertisements.
Parents/caregivers completed a brief web screening to determine initial eligibility. Parents who were preliminarily eligible were contacted to complete the Phone Screen, which involved a brief study overview and additional eligibility questions.
Following this, the Screening Visit (SV) and Baseline Visit (BV) were scheduled. Study measurements are detailed in Table 2. Parent/caregiver consent and child assent for the study was obtained in stages prior to the respective data collection: first for the web and phone screens, then for the screening visit andfull study. At the SV and BV, height and weight were measured, and participants completed questionnaires. A lifestyle interview was administered at SV to identify potential barriers to study participation. The interview ensured participants understood study protocol and were willing/able to take part. Following this visit, if the family remained interested and deemed eligible, they were randomized for enrollment. After randomization, the intervention commenced, and families were asked to complete assessment visits at month 6 (mid-point), month 12 (end-of-intervention), and month 18 (follow-up).
Table 2.
Data measurement and collection schedule
| Domain | Measurement (for Whom) | # of items | Respondenta | Phone Screenb | Screen Visit | BV | M1.5 | M6 | M12 | M18 |
|---|---|---|---|---|---|---|---|---|---|---|
| Relative Weight | Height | - | Child | X | X | X | X | X | X | |
| - | Parent | X | ||||||||
| Weight | - | Child | X | X | X | X | X | X | ||
| - | Parent | X | X | X | X | X | ||||
| Mental Health | Eating Disorder Symptoms (Parent/Child) | 6 | Parent | X | X | X | X | |||
| 6 | Child | X | X | X | X | |||||
| CESD-10 + 2 ASQ (Child) | 12 | Child | X | |||||||
| PHQ-9 (Parent) | 9 | Parent | X | |||||||
| GAD-7 (Parent) | 7 | Parent | X | |||||||
| PSC-17 (Child) | 17 | Parent | X | |||||||
| Family | CHAOS (Family) | 15 | Parent | X | ||||||
| FNPA (Family) | 20 | Parent | X | X | X | X | ||||
| Quality of Life | Experiences with Teasing (Child) | 12 | Child | X | ||||||
| Coping with Teasing (Child) | 7 | Child | X | X | X | X | ||||
| Sizing Them Up (Child) | 22 | Parent | X | X | X | X | ||||
| Pediatric Quality of Life (Child) | 23 | Child | X | X | X | X | ||||
| SF-12 (Parent) | 12 | Parent | X | X | X | X | ||||
| Motivation | Autonomy Support (Parent) | 3 + 3c | Parent | X | X | X | X | X | ||
| Self-Regulation (Parent) | 8 | Parent | X | X | X | X | ||||
| Late Study Measures | Change in Health History | 20 | Study Staff | X | X | X | ||||
| Acceptability | 12 | Parent | X | X | X | |||||
| 8 | Child | X | X | X | ||||||
| Medical Record | - | Study Staff | X | |||||||
| Other | Clinical Interview (Parent/Child) | 34 | Parent/Child | X | ||||||
| Demographics (P/C) | 29 | Parent | X |
Yellow indicates reporting by parent/child and blue indicates information collected by study staff.
PCP/Provider authorization to participate was needed between the Phone Screen and the Screening Visit.
eSOC + FBT families were asked 3 additional questions pertaining to their FBT coach.
Study data were collected and managed using Research Electronic Data Capture (REDCap), a secure, web-based application design to support data capture for research.29
Randomization and Blinding
The TEAM UP Data Coordinating Center (DCC) utilized the REDCap randomization module to randomly assign families to either eSOC or eSOC+FBT. Randomization was blocked within clinical practice using random block sizes and stratified by both sex and race (white and non-white). Data collection staff were blinded to the greatest extent possible; unblinding occurs rarely, e.g. when families unintentionally reveal their condition. Investigators not directly involved in supervising treatment delivery or providing medical oversight were blinded. Participating families were aware of their assignment, as were providers delivering treatment.
Description of Enhanced Standard of Care (eSOC)
All enrolled participants received eSOC, which was administered by the child’s PCP following the AAP Obesity Clinical Decision Support Chart and Next Steps resource manual.30–32 Prior to recruitment, participating PCPs were trained to the AMA pediatric obesity treatment recommendations33 that account for clinical practice capacity, motivation of the family, child’s physical and emotional development, and weight status.33 This training, organized by the AAP (see Appendix), occurred during a multisession tele-education learning collaborative, with curriculum developed by the AAP Institute for Healthy Childhood Weight in conjunction with the advisory groups. To build capacity among PCPs to deliver best-practice, specialized care, the Project Extension for Community Healthcare Outcomes (ECHO®) model was used to connect providers with experts and allow for case conferencing and peer support.34 In 2019, the initial clinical practices and providers participated in a live telehealth training of 8 sessions over 8 hours, with a requirement that providers attend at least 6 of the 8 sessions to participate as TEAM UP PCPs (20- to 30-minute didactic portions followed by 30-minute case conferencing). Clinical practices and providers who joined the trial after this initial series were provided access to the filmed recordings and watched at least 6 of the 8 sessions. After the core sessions, from 2019 to 2023, providers were offered ongoing monthly (and then bimonthly) optional 1-hour sessions as a group with AAP faculty and invited guest lecturers delivering didactic information related to obesity treatment and facilitating case conferencing. Providers engaged in a range of trainings based on their availability and interest.
In accordance with AMA guidelines, children initially received in-office (or telehealth, particularly during the COVID-19 pandemic) counseling from their PCP, and then based on response and motivation/readiness for change, received a higher level of care as needed. Following the AMA guidance on the staged approach to pediatric obesity treatment (see Figure 1) and depending on family availability/interest and child’s response to treatment, providers were asked to offer at least 6 but up to 21 eSOC visits over the course of the 12-month intervention at the provider’s discretion and family’s schedule. At these visits, providers assessed weight progress, child/family motivation and readiness to change; problem solved barriers to weight loss; and implemented dietary and physical activity goals and strategies to support behavior change.33
Figure 1.
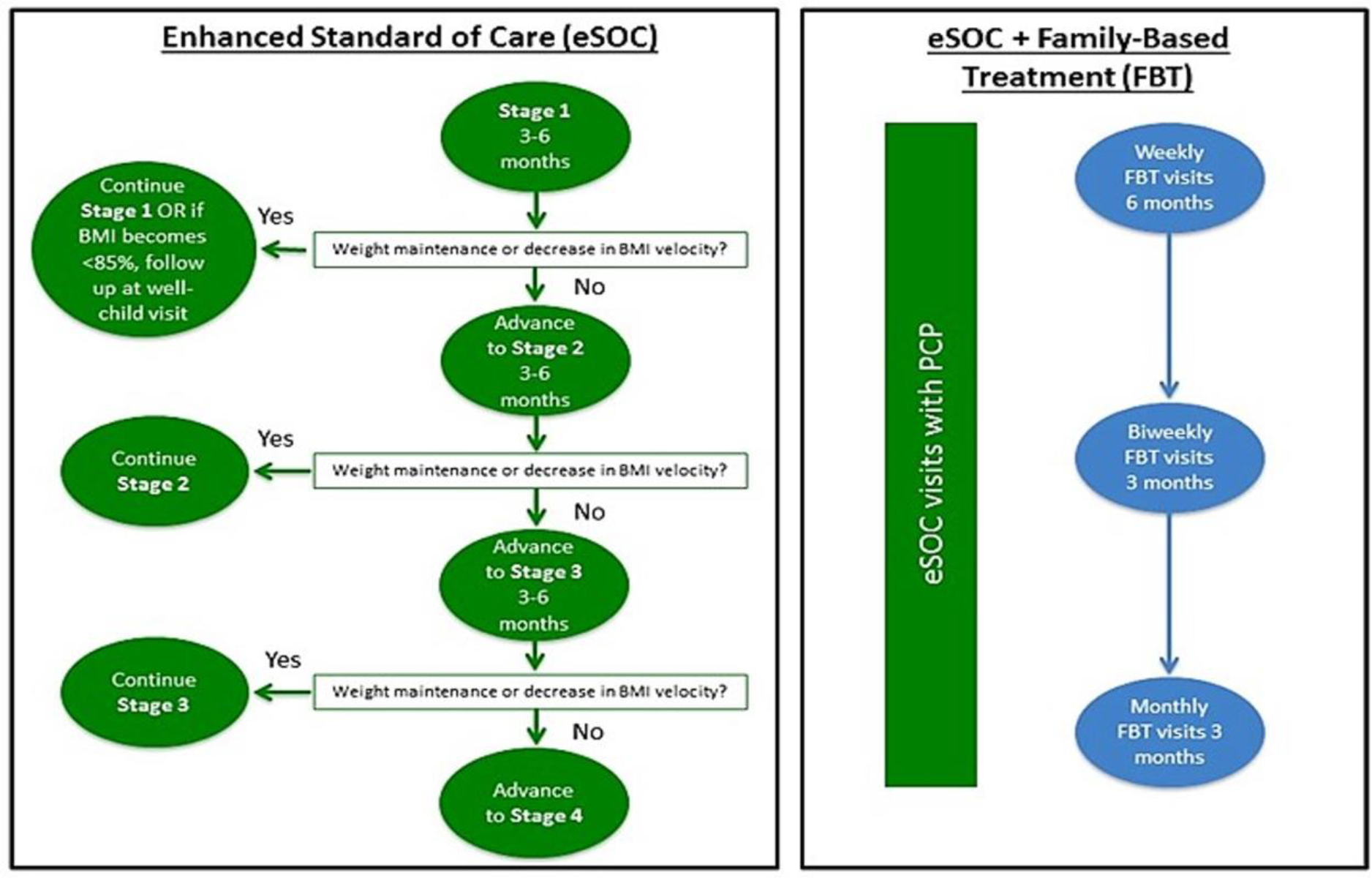
Flowchart illustrating how participants progressed through eSOC and eSOC+FBT.
Description of Family-Based Behavioral Treatment (FBT)
In conjunction with the eSOC and ongoing medical monitoring offered by the PCP, the families assigned to eSOC+FBT also engaged in FBT. FBT is a rigorously tested, multicomponent intervention that targets diet, activity, behavioral skills, parenting, and facilitation of support in family and peer environments.35–40 Coaches, who were existing practice staff wherever possible, were trained in the delivery of FBT (see Appendix). Following an initial workshop, coaches received ongoing training and supervision using the ECHO® model and methods, similar to the approach for eSOC providers described above, as well as weekly individual sessions with a study staff member experienced in FBT. The Training and Fidelity Core (TFC) at Washington University in St. Louis, MO provided oversight of supervisors for consistency across sites. Supervisors met as a group weekly to discuss areas of concern; they also performed monthly fidelity rating calibrations of audio recordings to ensure that consistency was maintained.
FBT included: 1) the Traffic Light Eating Plan, (i.e., a family-friendly method of color-coding foods to guide families toward the goal of consuming more low energy dense, high nutrient dense foods (GREEN), and fewer low nutrient, high energy dense foods (RED)). Children and their parents were provided individualized calorie goals and goals to reduce RED food intake and increase GREEN food intake; 2) the Traffic Light Activity Program also utilizes RED, YELLOW, and GREEN labels to categorize activities of different levels of caloric expenditure, to help increase physical activity and reduce sedentary behaviors. Parents and children were taught skills to decrease RED activity and increase GREEN activity; 3) behavioral strategies and parenting techniques, including stimulus control (e.g., parents were taught how to modify the home to create a healthier shared family environment), self-monitoring, goal setting, problem-solving, and finding substitutes for highly reinforcing foods. Parents were trained to use praise and positive reinforcement to shape and maintain their child’s healthy behaviors, as well as how to engineer healthy eating, activity, and sleep routines. Parents were encouraged to make changes in the same behaviors as their children, and to model these healthy behaviors and attitudes about behavior change; and 4) social facilitation focused on helping parents and children build supportive family and peer environments conducive to healthy weight-control behaviors and body esteem. Children were also coached in how to manage negative peer interactions (e.g., teasing) that hinder healthy behaviors and how to improve their ability to seek healthy peer-based alternatives to sedentary activities.38,39,41–43
FBT visits began as soon as feasible following the BV and concluded at the end of the one-year treatment window. FBT began with weekly visits for six months, then transitioned to biweekly FBT visits for three months, then monthly visits for three months, as feasible for the family. Families were seen in individual sessions, for approximately 30 to 50 minutes, that incorporated taking parent and child weights, review of eating and activity self-monitoring logs, review of weight change and connecting it to energy-balance behaviors, problem-solving and goal setting for the next meeting in relation to behavior change targets, and review of treatment handouts. FBT coaches used a “dashboard” to track information from their sessions in REDCap to manage treatment, charting, delivery, and oversight/supervision. This information was also used to calculate dose, fidelity, engagement, process data, and parent/caregiver and child behavioral changes. For care coordination, coaches communicated to the child’s PCP at least quarterly including patient progress, attendance, and any medical concerns.
Study Adaptations for COVID-19
The COVID-19 pandemic set off a national public health emergency in early 2020; it interrupted the study progress and constrained (in many cases closed recruitment and enrollment) study-related activities, with additional disruptions over time due to virus variants. The study adapted to these circumstances and resumed activities under COVID-19 precautions and safety protocols. Adaptations included offering flexibility for training/onboarding of PCPs to deliver eSOC; re-programming of REDCap to allow for a fully remote delivery from screening through end of study; training study personnel to utilize remote methods (online, video, phone) for treatment delivery, enrollment, and data collection; and purchasing and providing study data collection equipment (digital scale, metal tape measure, and carpenter’s square) to all participants for at-home height and weight measurements.
Primary and Secondary Outcome Measures
The primary outcome measure was child percent overweight, defined as .
Median BMI was normalized for child age and sex based on nationally representative data.44, 45 Secondary measures are listed below. See Table 2.
Physical Measurements
Study-provided equipment, as described above, was mailed to all participant homes, so that families were prepared should an assessment need to be completed remotely. A validation study of 37 families within the TEAM UP study indicated high concordance and reliability with no significant differences in height or weight collected remotely vs. in-person. Physical measurements were performed on child (primary outcome) and parent (secondary outcome).
Height (in-person).
Trained staff measured participants’ height twice to the nearest 0.1 cm using a Seca 213 portable stadiometer or equivalent in the PCP office, with a third measurement if first two differed by >0.3 cm.
Height (remote).
Families were sent written instructions with an instructional video prior to assessment for remote height measurements. Height information was collected following Centers for Disease Control (CDC) guidelines,47 with study staff observing via videoconferencing (exceptions were made occasionally, when families had faulty Wi-Fi or video equipment). Parents/caregivers were instructed to collect height twice to the nearest 0.1 cm for their child using the provided materials and instructions, with a third measurement if first two differed by >0.3 cm.
Weight (in-person).
Trained study staff measured participants’ weight twice without shoes to the nearest 0.1 kg using a Seca 876 medical digital scale in the PCP office, with a third measurement if the first two differed by >0.3kg.
Weight (remote).
Using the CDC guidelines for recording weight from home,47 participants used an Etekcity scale (model No. EB4473C). Weight was measured two times, with a third measurement if the first two differed by >0.1 kg. Remote weight measurements were observed by trained staff via videoconferencing whenever possible, with a third measurement if the first two differed by >0.3kg.
Child and Parent Report
Acceptability.
Children and parents/caregivers were asked to self-report on the acceptability of the intervention using the validated 8-item Client Satisfaction Questionnaire.48
Eating Disorder Screening and Monitoring.
Trained staff administered this 6-item interview-style measure assessing dietary restraint, weight and shape concerns within the last 28 days, and loss of control eating episodes and purging behaviors within the last 3 months, adapted from the validated Eating Disorder Examination Questionnaire.49,50
Child Report
Child Depression and Suicide Screening.
The 10-item Center for Epidemiological Studies Depression Scale Revised (CESD-R-10) was used as a self-report measure for child participants to screen for symptoms of depression during the past week. Suicidality in children was assessed using the 2-item self-report Ask Suicide-Screening Questions (ASQ).51 The study staff member administered the Columbia-Suicide Severity Rating Scale (C-SSRS)52 when there was elevated risk and followed the study-approved risk management procedures; imminent risk was treated as a psychiatric emergency.
Quality of life.
The Pediatric Quality of Life (PedsQL)53 is a 23-item self-report questionnaire that was used to assess physical, emotional, social, and school functioning in the past month.
Teasing.
History of experiences with weight-based teasing was measured using an adapted version of the Adolescent Experiences with Weight and Bullying self-report questionnaire.54 This questionnaire assessed type of bullying experienced (if any) and consequences experienced due to weight-based teasing. To measure the child’s ability to cope with teasing and to monitor teasing throughout the study, the 6-item problem-focused Adapted Coping with Teasing subscale of the Coping with Teasing Scale55 was used.
Parent/Caregiver Report
Parent/Caregiver Depression and Anxiety Screening. Parents/caregivers completed the 9-item self-report Patient Health Questionnaire (PHQ-9)56 to assess symptoms of depression and suicidality. Parents at elevated risk were administered the C-SSRS (for safety purposes, not an outcome); if elevated or imminent risk was confirmed, the staff member followed the study risk management procedures. Parents/caregivers also completed the General Anxiety Disorder-7 (GAD-7) self-report questionnaire to screen for symptoms of general anxiety.
Quality of Life.
Parents/caregivers completed the 12-Item Short form Survey (SF-12),57 an abbreviated version of the 36-item questionnaire, used to measure functional emotional and physical health and well-being over the last 4 weeks.
Motivation.
To examine their motivation to begin or continue eating a healthy diet and regularly engage in physical activity, parents/caregivers completed the 8-item Autonomous Self-Regulation subscale of the Treatment and Self-Regulation Questionnaire.58
Perceived support.
Parents/caregivers completed the Health Care Climate Questionnaire (HCCQ),59 a 15-item questionnaire used to measure perceived supportiveness from healthcare providers regarding health behavior change. Follow-up assessments at month 1.5 (sent by email) and then months 6, 12, and 18 asked about PCP (for all families) and FBT coaches (for those in the eSOC+FBT condition).
Household Chaos.
To measure environmental disorder in the home, parents/caregivers completed the 15-item Confusion, Hubbub, and Order Scale.60
Family Nutrition and Physical Activity.
Parents/caregivers completed the 20-item Family Nutrition & Physical Activity Screening Tool (FNPA)61 used to measure family environments and practices related to family meals, family eating practices, food choices, beverage choices, restriction/reward, screen time, healthy environment, family activity, child activity, and family schedule/sleep routine.
Demographics.
Demographics were assessed for descriptive and covariate analysis purposes. Parents/caregivers self-reported household income, and parent/child education level, medication use, sex, gender, and race/ethnicity. A validated 2-item measure62 was used to assess for food insecurity, and income volatility and predictability were measured with a 3-item questionnaire.63,64
Changes in Health History.
Parents/caregivers were interviewed to report potential adverse events and what, if any, weight-related medical visits the child attended outside of the primary care setting, with whom, for what duration, and the purpose of the visit(s). Adolescents (≥13 years) were also asked to report their own changes in health history.
Parent-Report on Child
Child Psychosocial Functioning.
Parents/caregivers completed the Pediatric Symptom Checklist-17 (PSC-17),65 an abbreviated version of the original 35-item scale to capture emotional and behavioral symptoms.
Impact of Weight on Child Functioning.
The Sizing Them Up66 questionnaire is a 22-item parent/caregiver report tool that was used to assess the impact of weight on the child’s health and daily functioning over the last month.
Provider Measures
Provider Survey.
Each PCP and FBT coach provided consent and then completed the Provider Survey at the beginning and end of their participation in the trial. This survey was adapted from the POWER67 and PROPEL68 weight loss trials and assessed demographics, clinical care, research activities, and knowledge about weight management practices. This survey utilized the provider acceptance subscale of the Evidence-Based Practice Attitude Scale,69 the weight bias subscale of the 14-item Fat Phobia Scale,70 provider competence,71 and provider intended uptake as measured by an adapted item from Scott.72
Other Study Measures
Parallel Medical Record Data.
A parallel effort of clinical and laboratory measurement collection was done utilizing EMR and/or paper medical charts at the participating clinics. A retrospective chart review covering the period of intervention and up to 2 years prior and 11.5 years after was conducted to assess changes in variables of interest. These EMR data are also used to supplement study-measured height and weight data in the case of a missed assessment visit.
Adverse Events.
At each assessment time point, parent/caregiver and child participants reported any unexpected health events that occurred during the duration of the study. The relatedness of the event to the study, expectedness, severity, and frequency of the event was reported to the DCC, and in the case of a serious adverse event was reviewed by the study medical investigators and reported to governing bodies as required.
eSOC Fidelity.
Medical record data were used to measure fidelity and treatment dose of eSOC, including frequency of follow-up visits scheduled and attended within the clinics and follow-up for specialist referrals and labs ordered. Recorded audits and practice-level changes in provider billing for obesity services were also assessed when available.
FBT Fidelity.
The Dashboard, mentioned above, was completed by FBT coaches for each session and used to measure the fidelity of FBT. Session audio recordings were randomly audited and rated by study supervisors.
Analytic Plan
All analyses adhere to the Methodology standards of the study’s main sponsor, the Patient-Centered Outcomes Research Institute (PCORI).73 Means and frequencies are tabulated to describe the participants, providers, and clinical practices, and to confirm no baseline differences by treatment arm among the participants. The primary analytic strategy for assessing intervention effects is a mixed model repeated-measures analysis of variance overall and within race and sex subgroups, using child percent overweight at each timepoint. To examine if the intervention impacts children and adolescents differently based on age, the variable age is tested as a moderator on the primary outcome using the Baron and Kenny method74 and bootstrapping methods.75 Primary and secondary endpoints are analyzed as continuous variables. In all analyses, we adjust for confounders including the practice site and provider using random effects and evaluated group-by-site interactions to determine whether the effectiveness of the intervention differs by site. Additional covariates include enrollment related to before or during the COVID-19 pandemic and number of trainings attended by PCPs, among others. Data are analyzed with SAS using the intent-to-treat principle.
Conclusion
TEAM UP is one of the largest pragmatic trials of an intensive health behavior lifestyle treatment program delivered for children and adolescents with obesity within primary care. Importantly, families and other partners informed the study design, recruitment materials, and measures, and provided ongoing input throughout each phase of study implementation. For pragmatism, the IHBLT program was imbedded within the primary care practice in conjunction with PCP-led counseling as recommended by the AMA and the AAP. All study decision making was based on trying to mimic, as closely as possible, what would happen in non-research clinical practice. Trial results inform the effectiveness of integrating IHBLT with the provider-led (eSOC) approach for changing children’s and parents’ relative weight outcomes as well as influence other patient-centered outcomes including psychosocial variables and relevant comorbid conditions. Broad eligibility criteria, a focus on clinical practices with a large proportion of Medicaid members, and a concerted effort to enroll racial and ethnic minority populations contribute to the potential generalizability of findings. Heterogeneity of treatment effects are examined to identify potential difference in effectiveness among sub-groups including between boys and girls and between White and non-White participants. The RE-AIM analysis provide in-depth examination of uptake, acceptability, and implementation, as well as likelihood of sustainability. TEAM UP provides timely, important results to inform the delivery of care and treatment options for children and adolescents with obesity.
Acknowledgements:
The following individuals and institutions constitute the TEAM UP Research Group: (*indicates principal investigator or director): COORDINATING CENTERS Washington University at St. Louis: Denise Wilfley*, Amy Braddock (University of Missouri), Angela Lima, Timothy McBride, Richard I. Stein, R. Robinson Welch, Janis Stoll. University of Rochester: Stephen Cook*, Anne-Marie Conn, Kristine DiBitetto, Kevin Fiscella, Geoffrey C. Williams. Pennington Biomedical Research Center: Amanda Staiano*, Robbie Beyl, Ricky Brock, Alyssa Button, Stewart Gordon, Lindsay Hall, Lauren N. Himel, William Johnson, Natalie Malek, Robert L. Newton Jr., Robert K. Singletary, Angelle Ullmer, Ava Zebrick. American Academy of Pediatrics: Alison Baker, Sandra Hassink, Jeanne Lindros, Victoria Rogers, Jeremiah Salmon.
The TEAM UP Research Group also acknowledges the tremendous involvement and contributions of the participating families, clinical practices, FBT coaches, eSOC healthcare providers, the external Data and Safety Monitoring Board, and our Advisory Board members especially: Family Advisory Board: Ava Zebrick, MS (Co-chair), Joe Nadglowski (Co-Chair), Melanee May, Bruce Hall, MD, PhD, Bill Michaels, Megan Betts, Tundra Alfred, Kytara Christophe, Anne Raggio, Nancy Hulslander, Vicky Hulslander. Provider Advisory Board: Sarah Hampl, MD (Chair), Sarah Barlow, MD, MPH, Lynn Bufka, PhD, Ihuoma Eneli, MD, MS, Ken Haller, MD, Susan McDaniel, PhD, Deborah Parra-Medina, PhD, MPH, Marsha Schofield, RD, MSN, Amy Braddock, MD, MSPH. Payer Advisory Board: Stewart Gordon, MD (Chair), Quinn Banquer, Jenny Bogard, MPH, Ravi Johar, MD, Timothy Kling, MD, FACOG, Timothy McBride, PhD, Samar Muzaffar, MD, MPH, Edmund Pezalla, MD, MPH, Laura Jean Shipley, MD, Laura Trunk, MD, MBA, Kim Tuck, RN. Evidence-Based Practice Advisory Board: Peter Katzmarzyk, PhD (Co-chair), Christie Befort, PhD (Co-chair), Sandra Hassink, MD, Elizabeth O’Connor, PhD, Asheley Cockrell Skinner, PhD, Susan Woolford, MD, MPH.
FUNDING
Research reported in this publication was funded through Patient-Centered Outcomes Research Institute® (PCORI®) Award PCS-2017C2-7542. The statements presented in this publication are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee. Additional funding was received from Blue Cross and Blue Shield of Louisiana and Louisiana Healthcare Connections. Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant # UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH); the Pennington Biomedical Research Center grant # U54 GM104940 funded by the NIH National Institutes of General Medical Sciences (NIGMS), Institutional Development Award Program Infrastructure for Clinical and Translational Research (IDeA-CTR); the University of Rochester CTSA award number UL1 TR002001 from the National Center for Advancing Translational Sciences; training grant # T32 HL 130357 provided by the National Heart, Lung and Blood Institute; and a NORC Center Grant # P30DK072476 titled “Nutrition and Metabolic Health Through the Lifespan” sponsored by NIDDK. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Appendix
Training of TEAM UP Primary Care Providers to Deliver enhanced Standard of Care
| eSOC Training Stage | Pre-Work |
Core Curriculum
Phase 1 |
Fidelity & Sustainability
Phase 2 |
Ongoing Engagement & Sustainability
Phases 3, 4 & 5 |
|---|---|---|---|---|
| Dates | February-April 2019 Or when the PCP joined the study |
April-July 2019 Or when the PCP joined the study |
September 2019-August 2020 | September 2020-February 2023 |
| Format | Self-paced modules and one-on-one introductions to faculty & staff | Utilized ECHO Methodology Monthly Virtual* Sessions with Case presentations |
Continued virtual* ECHO Quality improvement project: Multiple data cycles, PDSAs, Team reporting |
Virtual* learning collaboration Key topics & occasional case discussion Ongoing technical assistance Faculty and/or staff provided relevant case examples, where appropriate |
| Duration | Self-paced modules | 60 minutes | 60 minutes | 60 minutes |
| Key Topics Covered | Team Up Introductions Motivational Interviewing Welcome Call with Faculty & AAP Staff Pre-Project Survey ChangeTalk MI Simulations |
Introduction and Orientation to eSOC Pathophysiology Assessment & Management Practice Workflow, Coding & Billing The Provider Approach Weight Bias & Stigma Cultural Considerations Behavioral Counseling |
Follow Up Visits Developmental Approach Addressing Patient & Family Setbacks Talking with Patients and Families Obesity Care During the Pandemic Frontline Utilization Sustaining Your Practice Changes |
Obesity: A Complex Chronic Disease Goal Setting Using Rewards Obesity & COVID Enrolling Families in eSOC Self-Monitoring Opening the Conversation Obesity Coding & Billing Depression and Anxiety in Children w/Obesity Patient Engagement and Retention Identifying & Managing Pre-Diabetes Maintaining Treatment in Primary Care Multi-Disciplinary Treatment in Primary Care Role of Anti-Obesity Medications in Treatment |
Virtual session participation could be live (videoconferencing) or via recordings; live participation encouraged
Training of TEAM UP Family-based Behavioral Treatment (FBT) Coaches to Deliver FBT
| FBT Training Stage | Pre-Work |
Core ECHOs Required for training/FBT Certification |
Sustainability ECHOs Optional |
Supervision |
|---|---|---|---|---|
| Dates | June - November 2019 Or when the coach joined the study |
September-November 2019 | November2019-February 2020 Booster training ECHO in February 2021 |
September 2019-Currently ongoing |
| Format | 13-hour in-person training with live and recorded presentations Virtual training with pre recorded presentation on material FBT role play sessions Self-paced material review-review of lesson plans, handouts Book review- The Everyday Parenting Toolkit (Kazdin & Rotella, 2014), Childhood Obesity (Advances in Psychotherapy-Evidence-Based Practice; Wilfley, Best, Holland, & Van Buren, 2018) EMR/Data entry training Final Simulation review completed by trained staff |
Utilized ECHO Methodology Virtual* Sessions with Case presentations Weekly meetings for 1 month, bi weekly for 2 months |
Utilized ECHO Methodology Monthly |
Virtual* learning collaboration 2x/month (reduced to lx/month during final 6 months) Key topics & occasional case discussion, but did not follow ECHO format Ongoing technical assistance Faculty and/or staff provided relevant case examples, where appropriate |
| Duration | Self-paced modules | 60 minutes | 60 minutes | 60 minutes |
| Key Topics Covered | Family-Based Treatment Key Topics- Healthy Eating Physical Activity Routines Social Facilitation Maintenance & Relapse Prevention Nature and Treatment of Childhood Obesity Parenting Strategies |
“Dashboard” FBT EMR system Shaping Goals Self-Monitoring Rewards System Meal Planning Parenting Behaviors Teasing and Bullying Care Coordination |
Working with Families of Low Socio-economic Status Body Image Food Fussiness Patient Retention REDCap FBT Dashboard Cultural Adaptations Implicit Bias Social Influences Success post-TEAM UP |
Relevant program updates EMR / Data entry queries and questions Specific family issues and barriers Common topics that were discussed within individual supervision. Specific questions and cases coaches brought to the meeting |
Virtual session participation could be live (videoconferencing) or review didactic only via recordings; live participation encouraged
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing Interests: The authors have no competing interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hu K, Staiano AE. Trends in obesity prevalence among children and adolescents aged 2 to 19 years in the US from 2011 to 2020. JAMA Pediatrics. 2022;176(10):1037–1039. doi: 10.1001/jamapediatrics.2022.2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Defining childhood weight status. 2022. https://www.cdc.gov/obesity/basics/childhood-defining.html
- 3.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: Systematic review and meta-analysis. Obesity Reviews. 2016;17(2):95–107. doi: 10.1111/obr.12334 [DOI] [PubMed] [Google Scholar]
- 4.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. New England Journal of Medicine. 2015;373(14):1307–1317. doi: 10.1056/NEJMoa1502821 [DOI] [PubMed] [Google Scholar]
- 5.Mokhlesi B, Temple KA, Tjaden AH, et al. The association of sleep disturbances with glycemia and obesity in youth at risk for or with recently diagnosed type 2 diabetes. Pediatric Diabetes. 2019;20(8):1056–1063. doi: 10.1111/pedi.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: Epidemiology and treatment. Current Diabetes Reports. 2014;14(8):508. doi: 10.1007/s11892-014-0508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitaker BN, Fisher PL, Jambhekar S, et al. Impact of degree of obesity on sleep, quality of life, and depression in youth. Journal of Pediatric Health Care. 2018;32(2):e37–e44. doi: 10.1016/j.pedhc.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 8.Sutaria S, Devakumar D, Yasuda SS, Das S, Saxena S. Is obesity associated with depression in children? Systematic review and meta-analysis. Archives of Disease in Childhood. 2019;104(1):64–74. doi: 10.1136/archdischild-2017-314608 [DOI] [PubMed] [Google Scholar]
- 9.Staiano AE, Morrell M, Hsia DS, Hu G, Katzmarzyk PT. The burden of obesity, elevated blood pressure, and diabetes in uninsured and underinsured adolescents. Metabolic Syndrome and Related Disorders. 2016;14(9):437–441. doi: 10.1089/met.2016.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3)doi: 10.1542/peds.2017-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampl SE, Hassink SG, Skinner AC, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023:e2022060640. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for eight management in children and adolescents: Evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(23):2427–2444. [DOI] [PubMed] [Google Scholar]
- 13.Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial. JAMA. 2007;298(14):1661–1673. doi: 10.1001/jama.298.14.1661 [DOI] [PubMed] [Google Scholar]
- 14.Skinner AC, Staiano AE, Armstrong SC, et al. Appraisal of clinical care practices for child obesity treatment. Part I: Interventions. Pediatrics. 2023; 151(2):e2022060642. doi: 10.1542/peds.2022-060642. [DOI] [PubMed] [Google Scholar]
- 15.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120 Suppl 4:S164–92. doi: 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 16.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: Evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(23):2427–2444. doi: 10.1001/jama.2017.0332 [DOI] [PubMed] [Google Scholar]
- 17.Fassbender JE, Wallace SL, Tan-Torres S. Provider views of the feasibility and utility of lifestyle obesity treatment in primary care: Insights from the Think Health! study. European Journal for Person Centered Healthcare. 2015;3(1):77–82. [Google Scholar]
- 18.Epstein LH, Wilfley DE, Kilanowski C, et al. Family-based behavioral treatment for childhood obesity implemented in pediatric primary care: A randomized clinical trial. JAMA. 2023;329(22):1947–1956. doi: 10.1001/jama.2023.8061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eichen DM, Rhee KE, Strong DR, Boutelle KN. Impact of race and ethnicity on weight-loss outcomes in pediatric family-based obesity treatment. Journal of Racial and Ethnic Health Disparities. 2020;7(4):643–649. doi: 10.1007/s40615-019-00694-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davison GM, Fowler LA, Ramel M, et al. Racial and socioeconomic disparities in the efficacy of a family-based treatment programme for paediatric obesity. Pediatric Obesity. 2021;16(10):e12792. doi: 10.1111/ijpo.12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prioste A, Fonseca H, Sousa P, Gaspar P, Machado MdC. Cross-sectional study showed psychosocial variables, gender and family involvement played an important role in an adolescent weight management programme. Acta Paediatrica. 2017;106(1):105–111. doi: 10.1111/apa.13616 [DOI] [PubMed] [Google Scholar]
- 22.Chang E, Varghese M, Singer K. Gender and sex differences in adipose tissue. Current Diabetes Reports. 2018;18(9):69. doi: 10.1007/s11892-018-1031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batch BC, Goldstein K, Yancy WS Jr., et al. Outcome by gender in the Veterans Health Administration Motivating Overweight/Obese Veterans Everywhere Weight Management Program. Journal of Women’s Health. 2018;27(1):32–39. doi: 10.1089/jwh.2016.6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemon SC, Rosal MC, Zapka J, Borg A, Andersen V. Contributions of weight perceptions to weight loss attempts: Differences by body mass index and gender. Body Image. 2009;6(2):90–96. doi: 10.1016/j.bodyim.2008.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutelle KN, Cafri G, Crow SJ. Parent predictors of child weight change in family based behavioral obesity treatment. Obesity. 2012;20(7):1539–1543. doi: 10.1038/oby.2012.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldschmidt AB, Best JR, Stein RI, Saelens BE, Epstein LH, Wilfley DE. Predictors of child weight loss and maintenance among family-based treatment completers. Journal of Consulting and Clinical Psychology. 2014;82(6):1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasgow RE. RE-AIMing research for application: Ways to improve evidence for family medicine. The Journal of the American Board of Family Medicine. 2006;19(1):11–19. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy BM, Davison G, Fowler LA, et al. Perceptions of a pragmatic family-centered approach to childhood obesity treatment. Ochsner Journal. 2021;21(1):30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Academy of Pediatrics. Pediatric obesity clinical decision support chart. Elk Grove Village, IL: American Academy of Pediatrics. 2008:732–7. [Google Scholar]
- 31.Fanburg JT, Rogers VW, Dedekian MA, Cooke E, Anand SG, Homer CJ. Next steps: A practitioner’s guide of themed follow-up visits to help patients achieve a healthy weight. American Academy of Pediatrics; 2014. [Google Scholar]
- 32.American Academy of Pediatrics. Obesity Prevention, Assessment, and Treatment Algorithm. https://downloads.aap.org/AAP/PDF/algorithm_brightfutures_032819.pdf?_ga=2.213841940.1052762415.1671117296-1461408389.1663186636. [Google Scholar]
- 33.Barlow SE, Committee E. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120(Supplement_4):S164–S192. [DOI] [PubMed] [Google Scholar]
- 34.Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Academic Medicine: Journal of the Association of American Medical Colleges. 2007;82(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davison GM, Monocello LT, Lipsey K, Wilfley DE. Evidence base update on behavioral treatments for overweight and obesity in children and adolescents. Journal of Clinical Child & Adolescent Psychology. 2023;52(5):589–603. doi: 10.1080/15374416.2023.2251164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilfley DE, Tibbs TL, Van Buren D, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: A meta-analytic review of randomized controlled trials. Health Psychology. 2007;26(5):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epstein LH, Wilfley DE, Kilanowski C, et al. Family-based behavioral treatment for childhood obesity implemented in pediatric primary care: A randomized clinical trial. JAMA. 2023;329(22):1947–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial. JAMA. 2007;298(14):1661–1673. [DOI] [PubMed] [Google Scholar]
- 39.Wilfley DE, Saelens BE, Stein RI, et al. Dose, content, and mediators of family-based treatment for childhood obesity: A multisite randomized clinical trial. JAMA Pediatrics. 2017;171(12):1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA. 1990;264(19):2519–2523. [PubMed] [Google Scholar]
- 41.Epstein LH, Schechtman KB, Kilanowski C, et al. Implementing family-based behavioral treatment in the pediatric primary care setting: Design of the PLAN study. Contemporary Clinical Trials. 2021;109:106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epstein LH, Valoski AM, Vara LS, et al. Effects of decreasing sedentary behavior and increasing activity on weight change in obese children. Health Psychology. 1995;14(2):109. [DOI] [PubMed] [Google Scholar]
- 43.Epstein LH, Paluch RA, Beecher MD, Roemmich JN. Increasing healthy eating vs. reducing high energy-dense foods to treat pediatric obesity. Obesity. 2008;16(2):318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. National Health Statistics Report. 2013;(63):1–3. [PubMed] [Google Scholar]
- 45.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: Methods and development. Vital Health Stat Series 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 46.Button AM, Staiano AE, Beyl RA, et al. Validation of remote child weight and height measurements within a weight management trial. Obesity (Silver Spring). 2023;doi: 10.1002/oby.23972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. Measuring children’s height and weight accurately at home (2021). Available at http://medbox.iiab.me/modules/en-cdc/www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/measuring_children.html/
- 48.Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: Development of a general scale. Evaluation and Program Planning. 1979;2(3):197–207. [DOI] [PubMed] [Google Scholar]
- 49.Goldschmidt AB, Doyle AC, Wilfley DE. Assessment of binge eating in overweight youth using a questionnaire version of the Child Eating Disorder Examination with Instructions. International Journal of Eating Disorders. 2007;40(5):460–7. doi: 10.1002/eat.20387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the eating disorder examination with children: A pilot study. International Journal of Eating Disorders. 1996;19(4):391–7. doi: [DOI] [PubMed] [Google Scholar]
- 51.Horowitz LM, Bridge JA, Teach SJ, et al. Ask Suicide-Screening Questions (ASQ): A brief instrument for the pediatric emergency department. Archives of Pediatrics and Adolescent Medicine. 2012;166(12):1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry. 2011;168(12):1266–77. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Medical Care. 2001:800–812. [DOI] [PubMed] [Google Scholar]
- 54.Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: Bullying experiences of weight loss treatment–seeking youth. Pediatrics. 2013;131(1):e1–e9. [DOI] [PubMed] [Google Scholar]
- 55.Faith MS, Leone MA, Ayers TS, Heo M, Pietrobelli A. Weight criticism during physical activity, coping skills, and reported physical activity in children. Pediatrics. 2002;110(2 Pt 1):e23. [DOI] [PubMed] [Google Scholar]
- 56.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ware JE Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996:220–233. [DOI] [PubMed] [Google Scholar]
- 58.Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Education Research. 2007;22(5):691–702. [DOI] [PubMed] [Google Scholar]
- 59.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. Journal of Personality and Social Psychology. 1996;70(1):115. [DOI] [PubMed] [Google Scholar]
- 60.Matheny AP Jr, Wachs TD, Ludwig JL, Phillips K. Bringing order out of chaos: Psychometric characteristics of the confusion, hubbub, and order scale. Journal of Applied Developmental Psychology. 1995;16(3):429–444. [Google Scholar]
- 61.Ihmels MA, Welk GJ, Eisenmann JC, Nusser SM. Development and preliminary validation of a Family Nutrition and Physical Activity (FNPA) screening tool. International Journal of Behavioral Nutrition and Physical Activity. 2009;6(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hager ER, Quigg AM, Black MM, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126(1):e26–e32. [DOI] [PubMed] [Google Scholar]
- 63.Financial Industry Regulatory Authority I. National Financial Capability Study 2015. Available at https://finrafoundation.org/knowledge-we-gain-share/nfcs/about-nfcs [Google Scholar]
- 64.The Social Policy Institute. 2017 Household Financial Survey. In. St. Louis, MO: Washington University in St. Louis; 2017. [Google Scholar]
- 65.Murphy JM, Bergmann P, Chiang C, et al. The PSC-17: Subscale scores, reliability, and factor structure in a new national sample. Pediatrics. 2016;138(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Modi AC, Zeller MH. Validation of a parent-proxy, obesity-specific quality-of-life measure: Sizing them up. Obesity. 2008;16(12):2624–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennett WL, Wang N-Y, Gudzune KA, et al. Satisfaction with primary care provider involvement is associated with greater weight loss: Results from the practice-based POWER trial. Patient Education and Counseling. 2015;98(9):1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katzmarzyk PT, Martin CK, Newton RL Jr, et al. Promoting Successful Weight Loss in Primary Care in Louisiana (PROPEL): rationale, design and baseline characteristics. Contemporary Clinical Trials. 2018;67:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aarons GA. Mental health provider attitudes toward adoption of evidence-based practice: The Evidence-Based Practice Attitude Scale (EBPAS). Mental Health Services Research. 2004;6(2):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bacon JG, Scheltema KE, Robinson BE. Fat phobia scale revisited: The short form. International Journal of Obesity. 2001;25(2):252–257. [DOI] [PubMed] [Google Scholar]
- 71.Williams GC, Levesque C, Zeldman A, Wright S, Deci EL. Health care practitioners’ motivation for tobacco-dependence counseling. Health Education Research. 2003;18(5):538–553. [DOI] [PubMed] [Google Scholar]
- 72.Scott SD, Plotnikoff RC, Karunamuni N, Bize R, Rodgers W. Factors influencing the adoption of an innovation: An examination of the uptake of the Canadian Heart Health Kit (HHK). Implementation Science. 2008;3(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.The PCORI methodology report. Available at https://www.pcori.org/research/about-our-research/research-methodology/pcori-methodology-report
- 74.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173. [DOI] [PubMed] [Google Scholar]
- 75.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, and Computers. 2004;36(4):717–731. [DOI] [PubMed] [Google Scholar]


