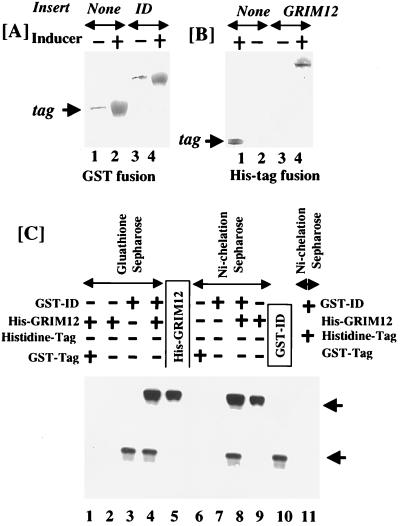
FIG. 13.
Binding of purified ID to GRIM-12 (TR) in vitro. ID and GRIM-12 were expressed as GST and histidine-tagged fusion proteins in E. coli. Pure proteins were obtained after two rounds of chromatography on glutathione-Sepharose or Ni-chelation-Sepharose columns. (A and B) Silver-stained SDS-PAGE gels (10% polyacrylamide) after purification of these proteins. Overexpression of fusion protein was performed by treating the cells with an inducer (IPTG). (C) Equimolar amounts of purified proteins were preincubated with each other before being subjected to chromatography on the indicated column. The presence or absence of the proteins in the incubation mixture is indicated by + and −, respectively. Affinity matrices used for separation after protein interaction are indicated above the arrows. After elution, the samples were loaded and separated by SDS-PAGE (10% polyacrylamide). Pure proteins (one-fifth of those in other lanes), without passing over the affinity matrices, were loaded in lanes 5 and 10. After separation, the samples were Western blotted with a TR-specific antibody. Western blotting was used to detect any nonspecific interactions between tags and fusion protein.