Abstract
Background
The synaptic hypothesis is an influential theory of the pathoetiology of schizophrenia (SCZ), which is supported by the finding that there is lower uptake of the synaptic terminal density marker [11C]UCB-J in patients with chronic SCZ than in control participants. However, it is unclear whether these differences are present early in the illness. To address this, we investigated [11C]UCB-J volume of distribution (VT) in antipsychotic-naïve/free patients with SCZ who were recruited from first-episode services compared with healthy volunteers.
Methods
Forty-two volunteers (SCZ n = 21, healthy volunteers n = 21) underwent [11C]UCB-J positron emission tomography to index [11C]UCB-J VT and distribution volume ratio in the anterior cingulate, frontal, and dorsolateral prefrontal cortices; the temporal, parietal and occipital lobes; and the hippocampus, thalamus, and amygdala. Symptom severity was assessed in the SCZ group using the Positive and Negative Syndrome Scale.
Results
We found no significant effects of group on [11C]UCB-J VT or distribution volume ratio in most regions of interest (effect sizes from d = 0.0–0.7, p > .05), with two exceptions: we found lower distribution volume ratio in the temporal lobe (d = 0.7, uncorrected p < .05) and lower VT/fp in the anterior cingulate cortex in patients (d = 0.7, uncorrected p < .05). The Positive and Negative Syndrome Scale total score was negatively associated with [11C]UCB-J VT in the hippocampus in the SCZ group (r = −0.48, p = .03).
Conclusions
These findings indicate that large differences in synaptic terminal density are not present early in SCZ, although there may be more subtle effects. When taken together with previous evidence of lower [11C]UCB-J VT in patients with chronic illness, this may indicate synaptic density changes during the course of SCZ.
Keywords: Antipsychotic-free, Positron emission tomography, Schizophrenia, SV2A, Synaptic density, UCB-J
SEE COMMENTARY ON PAGE 605
Multiple lines of evidence suggest that synaptic dysfunction plays a central role in schizophrenia (SCZ) pathogenesis. Initial reports concerned postmortem evidence of lower dendritic spine density (1,2), lower synaptic protein and messenger RNA levels (3), and genetic evidence (4, 5, 6). Recently, our group reported in vivo evidence for lower synaptic terminal density indexed using [11C]UCB-J imaging in patients with chronic SCZ than in healthy control participants (7). These findings were subsequently replicated by a separate group (8).
However, because the [11C]UCB-J imaging studies were conducted in patients with chronic SCZ, it is unknown whether synaptic deficits emerge early in the course of illness or develop later during the illness.
Therefore, we conducted a clinical imaging study using [11C]UCB-J positron emission tomography (PET) to test the hypothesis that [11C]UCB-J volumes of distribution (VT) would be lower in patients with early-course SCZ than in control participants in brain regions where evidence for lower synaptic terminal density has been reported in patients with chronic SCZ (3,7,8). We also tested the hypothesis that [11C]UCB-J VT would be inversely associated with symptom severity.
Methods and Materials
The London-West London & GTAC Research Ethics Committee, United Kingdom (reference: 16/LO/1941) approved the study protocol. The Administration of Radioactive Substances Advisory Committee, United Kingdom, approved the administration of radioactive material. We obtained written informed consent from all volunteers before their participation in the study, which was conducted in accordance with the Declaration of Helsinki (1996).
We recruited 42 volunteers (21 healthy volunteers [HVs] through public advertisement and 21 patients). PET and clinicodemographic data for 17 healthy volunteers [in analyses in (7,9)], but none of the SCZ group, have been reported previously. Inclusion criteria for all volunteers were 18 to 65 years of age, capacity to consent, and a normal blood coagulation test.
Patients were recruited from London first-episode psychosis services. Inclusion criteria were meeting DSM-5 criteria for SCZ and being antipsychotic-naïve or free from antipsychotic medication for at least 4 weeks before [11C]UCB-J imaging. HVs had to have no history of a mental disorder or family history of SCZ (see Supplemental Methods for exclusion criteria).
Clinical Assessments
The Structured Clinical Interview for DSM-5 and the Positive and Negative Syndrome Scale (PANSS) (10) were administered. Illness duration was determined as the time from each patient’s first psychotic symptoms. See Supplemental Methods for additional information.
Magnetic Resonance Imaging
All subjects underwent structural magnetic resonance imaging (MRI) to facilitate the anatomical delineation of regions of interest (ROIs) (see Supplemental Methods).
PET Acquisition and Analysis
PET Imaging
Following a low-dose computed tomography scan for attenuation and scatter correction, subjects received a microdose of [11C]UCB-J (≤300 MBq) as a smooth bolus injection via an intravenous cannula for 20 seconds. A Biograph 6 HiRez PET-CT scanner (Siemens) was used to acquire PET data for 90 minutes.
Arterial Blood Sampling
Radial arterial blood samples were collected throughout the PET scan to measure the arterial input function as has been detailed elsewhere (11). Briefly, a continuous automatic blood sampling system was used to measure whole-blood activity for the first 15 minutes (Allogg AB) (see Supplemental Methods for details).
Image Analysis
Processing and modeling were conducted using MIAKAT version 4.3.7 (http://www.miakat.org/MIAKAT2/index.html), which was implemented in MATLAB (version R2018b; The MathWorks, Inc.) with functions from FSL (version 5.0.10; FMRIB) and SPM12 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm).
Each subject’s MRI underwent brain extraction using FSL and gray matter segmentation and rigid-body coregistration to a standard reference space (12) using SPM12 as implemented via MIAKAT. The template brain image and associated Clinical Imaging Centre atlas (13) were then warped nonlinearly to the subject’s MRI. The frontal cortex (FC), anterior cingulate cortex (ACC), and hippocampus were defined as primary ROIs based on previous imaging and postmortem evidence for lower synaptic protein levels in these regions (3,7, 8, 9). We used the same atlas to define the occipital, parietal, and temporal lobes, dorsolateral prefrontal cortex, thalamus, and amygdala as exploratory ROIs because they have been implicated in SCZ pathophysiology (7,8,14, 15, 16). The centrum semiovale (CS) ROI was generated from the automated anatomical labeling template (17) according to parameters defined for its use as a reference region to estimate nondisplaceable [11C]UCB-J binding (18).
PET images were registered to each subject’s MRI and motion corrected through frame-to-frame rigid-body registration using the 14th frame (acquired 9–11 minutes after injection) as the reference frame. The extent of motion was assessed during the scan, and registration parameter plots were derived. Total movement was defined as total frame-by-frame Euclidean distance, which was estimated from frame realignment during motion correction. Time activity curves were generated for each ROI.
Regional time activity curves and arterial input function data were analyzed together using the 1-tissue compartment model, which produces reliable [11C]UCB-J VT estimates (11,19).
Gray matter masks were applied to ROIs within MIAKAT to extract regional gray matter VT. Regional distribution volume ratio (DVR) was obtained using the CS as a pseudoreference region (11,18), thus deriving DVR as a ratio of ROI VT to CS VT.
Sample Size and Power Calculation
We calculated the minimum sample size needed to test our primary hypothesis using G∗power version 3.1.9.3 (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower). Previous studies identified lower [11C]UCB-J binding in patients with SCZ than in control participants, with effect sizes around 0.9 or greater in our primary ROIs (7,8). The power calculation indicated that a minimum sample size of 21 subjects per group would have more than 80% power to detect a significant group difference in [11C]UCB-J binding with Cohen’s d ≥ 0.9.
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism version 8.00 for Mac (GraphPad Software, http://www.graphpad.com), IBM SPSS Statistics version 25 and RStudio Version 1.1.456 (RStudio Team [2016], RStudio, Inc., http://www.rstudio.com/). We tested for normality of distribution using the Shapiro-Wilk test. When data were normally distributed, we used two-way repeated measures analysis of variance to test group and ROI effects. We used planned independent sample t tests (two-tailed) to test the effect of group on VT at each ROI, applying a false discovery rate (FDR) correction with Q = 5% to limit false discoveries when performing multiple group comparisons (20). When data were not normally distributed, we used nonparametric analyses to test for group effects. We assessed group differences in clinicodemographic variables using independent sample t tests, χ2 tests, and Kolmogorov-Smirnov tests for normally distributed, categorical, and nonparametric data, respectively. We tested whether there were significant associations between VT and PANSS total scores using Pearson’s product-moment correlation coefficients for normally distributed data and Spearman’s rank correlations for non-normally distributed data. When an association was significant, we conducted exploratory analyses to test whether there were associations between VT and PANSS positive, negative, and general subscale scores.
For comparisons with previous studies, we conducted exploratory analyses using the same statistical approach to assess whether there were significant alterations in SCZ using [11C]UCB-J VT in other ROIs and using [11C]UCB-J DVR as the outcome measure instead of VT.
Exploratory voxelwise whole-brain analyses were conducted using SPM12 to determine whether there were alterations in [11C]UCB-J VT that were not detected by the ROI analysis (see Supplemental Methods).
Results
Forty-two volunteers (n = 21 HVs and n = 21 with SCZ) completed the study. There were no significant group differences in age, sex, ethnicity, proportion of current smokers, or cannabis use during the past month or in [11C]UCB-J injected activity, injected cold mass, specific radioactivity, minimum purity, or plasma-free fraction (fp) (Table 1).
Table 1.
Clinicodemographic and Imaging Variables in the HV and SCZ Groups
| HV | SCZ | Test Statistic | p | |
|---|---|---|---|---|
| Age, Years | 30.86 (1.90) [20–49] | 26.52 (1.74) [18–48] | z = 1.08 | .19 |
| Sex, Female/Male | 5/16 | 4/17 | χ21 = 0.14 | .71 |
| Ethnicity, Asian/Black/Other/White | 3/5/1/12 | 4/11/2/4 | χ23 = 6.73 | .08 |
| Current Smoker | 5 | 7 | χ21 = 0.47 | .49 |
| Cannabis Users Within the Last Month | 1 | 2 | χ21 = 0.36 | .55 |
| Activity Injected, MBq | 262.96 (4.94) [213.47–295.90] | 221.24 (12.84) [107.20–280.46] | z = 1.23 | .10 |
| Injected Mass, μg | 3.11 (0.22) [1.62–4.67] | 3.18 (0.35) [1.12–8.27] | z = 0.46 | .98 |
| Specific Radioactivity, GBq/μM | 30.41 (2.50) [15.27–51.84] | 25.64 (2.21) [10.37–43.76] | t40 = 1.43 | .16 |
| Minimum Purity Fraction | 99.97 (0.03) [99.34–100] | 100 (0.00) [100–100] | z = 0.15 | >.99 |
| [11C]UCB-J Plasma-Free Fraction | 0.24 (0.005) [0.20–0.29] | 0.27 (0.007) [0.23–0.38] | z = 1.23 | .10 |
| Total Motion During PET Scan, mm | 23.30 (5.87) [1.24–92.85] | 22.50 (6.12) [0.60–120.57] | z = 0.77 | .59 |
| Illness Duration, Years | – | 2.67 (0.46) [1–9] | – | – |
| Drug-Free Interval, Days | – | 180.42 (27.71) [41–448] | – | – |
Values are mean (SEM) [range] or n.
HV, healthy volunteer; PET, positron emission tomography; SCZ, schizophrenia.
None of the SCZ volunteers were taking antipsychotic medication. Two were antipsychotic-naïve, and 19 had taken antipsychotic medication previously, with a mean (SEM) interval of 180.42 (27.71) days and a minimum of 41 days between the most recent antipsychotic dose and [11C]UCB-J imaging. None of the SCZ volunteers had comorbid DSM-5 psychiatric diagnoses.
[11C]UCB-J VT Across Groups in Primary ROIs
Data were normally distributed in each ROI in each group. There was a significant effect of ROI (F2,40 = 609.4, p < .0001) but not of group (F1,20 = 0.09, p = .77; even with age as a covariate [F1,39 = 0.68, p = .41]) on [11C]UCB-J VT (Figure 1). Post hoc analyses with FDR adjustment revealed no significant group differences in any ROI (Figure 1; Table S1).
Figure 1.
[11C]UCB-J volume of distribution (VT) in the frontal cortex, anterior cingulate cortex (ACC), and hippocampus by group. Orange squares indicate the healthy volunteer (HV) group (n = 21); green dots indicate the schizophrenia (SCZ) group (n = 21). [11C]UCB-J VT was not significantly altered in the SCZ group compared with the HV group in any region of interest (ROI). Horizontal bar indicates mean; error bars indicate standard error of the mean.
Relationship Between [11C]UCB-J VT and Symptom Severity
In the SCZ group, mean (SEM) PANSS scores were as follows: total score, 65.29 (3.29); positive, 17.05 (1.24); negative, 17.81 (0.95); and general, 30.43 (1.81). In the SCZ group, there was a significant negative relationship between hippocampal [11C]UCB-J VT and the PANSS total score (r = −0.48, p = .03) (Figure 2; Table S2) and a nonsignificant trend toward a negative relationship for VT with positive (r = −0.42, p = .06) and general (r = −0.42, p = .06) but not negative PANSS subscale scores (Figures S1–S3). There were no significant relationships between FC or ACC [11C]UCB-J VT and PANSS total scores (Figures S4, S5; Table S2).
Figure 2.
Significant negative association between hippocampal [11C]UCB-J volume of distribution (VT) and Positive and Negative Syndrome Scale (PANSS) total score.
Exploratory Analysis of [11C]UCB-J VT/fp in Primary ROIs
Given the finding of numerically greater fp in the SCZ group than in the HV group, we calculated VT/fp as an alternative outcome measure to correct for differences in fp. There was a significant effect of ROI (F2,67 = 722.07, p < .0005) and of the group-by-ROI interaction (F2,67 = 3.83, p = .03) but not of group (F1,40 = 3.91, p = .06) on [11C]UCB-J VT/fp. Post hoc analyses revealed that VT/fp was significantly lower in the SCZ group in the ACC [mean (SEM) HV = 91.71 (1.93), SCZ = 83.79 (2.70), t40 = 2.38, p = .02, d = 0.7], although this finding did not survive FDR adjustment for multiple comparisons, and VT/fp was not significantly altered in the FC or hippocampus (Table S3).
Exploratory Analysis of [11C]UCB-J VT in Other Regions
There was a significant effect of ROI (F5,100 = 135.9, p < .0001) but not group (F1,20 = 0.04, p = .85), even with age as a covariate (F1,39 = 0.86, p = .34), on [11C]UCB-J VT in the exploratory ROIs.
Whole-Brain Analyses
A voxelwise whole-brain analysis showed that there were no significant differences between patients and control participants in [11C]UCB-J VT (familywise error–corrected p < .05), and this remained the case even when we used a liberal threshold (uncorrected p < .001).
[11C]UCB-J VT in the CS
Data were not normally distributed in the SCZ group. There was no significant group difference in mean (SEM) [11C]UCB-J VT in the CS [HV = 5.54 (0.13); SCZ = 6.34 (0.37); Kolmogorov-Smirnov z = 0.93, p = .36] (Table S1; Figure S6).
[11C]UCB-J DVR Across Groups
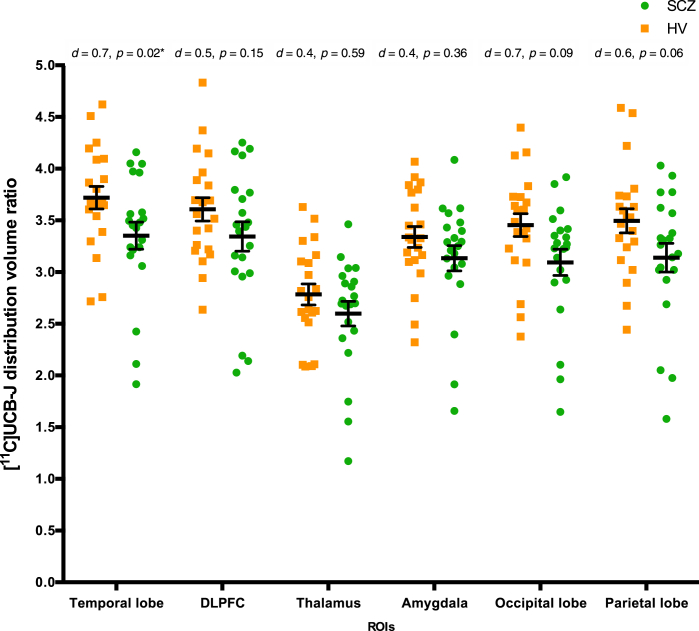
The mean [11C]UCB-J DVR was significantly lower in the SCZ group than in the HV group in the temporal lobe (Kolmogorov-Smirnov z = 1.54, p = .02, Cohen’s d = 0.7) (Figure 3), although this finding did not survive FDR adjustment for multiple comparisons. DVR was not significantly different between groups in any other regions, although values were numerically lower in the SCZ group than in the HV group (Supplemental Results; Tables S4 and S5; Figure 3; Figure S7).
Figure 3.
Mean [11C]UCB-J distribution volume ratio in the occipital, parietal, and temporal lobes; the dorsolateral prefrontal cortex (DLPFC); and the thalamus and amygdala, by group. Orange squares indicate the healthy volunteer (HV) group (n = 21); green dots indicate the schizophrenia (SCZ) group (n = 21). [11C]UCB-J distribution volume ratio was not significantly altered in any region of interest (ROI) following adjustment for multiple comparisons. ∗ indicates p value that did not survive false discovery rate adjustment. Error bars indicate standard error of the mean.
Relationship Between [11C]UCB-J DVR and Symptom Severity
In the SCZ group, there were no significant relationships between [11C]UCB-J DVR in the FC, ACC, or hippocampus and PANSS scores (Table S6). Given the exploratory finding of lower [11C]UCB-J DVR in the temporal lobe in the SCZ group, we also tested relationships between [11C]UCB-J DVR and PANSS scores in this region but found no significant relationships (Table S6).
Relationship Between [11C]UCB-J and Duration of Antipsychotic-Free Interval
There were no significant relationships between the duration of the interval since the most recent antipsychotic treatment and [11C]UCB-J VT or DVR in any ROI (Supplemental Results).
Discussion
The main finding of this study is that [11C]UCB-J VT, an in vivo marker of synaptic vesicle glycoprotein 2A (SV2A) levels and a proxy marker of presynaptic terminal density, is not significantly different between early-course SCZ and HV groups across the brain. However, hippocampal [11C]UCB-J VT is negatively associated with total PANSS score.
Our exploratory analyses using alternative outcome measures found evidence for lower DVR in the temporal lobe and lower VT/fp in the ACC in patients with early-course SCZ than in HVs, although neither finding survived correction for multiple comparisons. We also found trends toward lower DVR in the FC, ACC, and occipital and parietal lobes in patients with early-course SCZ than in HVs. [11C]UCB-J DVR data show lower variability than VT data (7). The increased sensitivity of the DVR approach to detect group differences could explain why there were trends for group differences in DVR but not VT. Another consideration is that DVR adjusts for nonspecific binding, while VT indexes total tracer distribution in the tissue. Thus, trends toward lower DVR and VT/fp values in patients could reflect lower SV2A levels that are masked in the VT data by nonspecific binding, subtle fp differences, and/or the lower sensitivity of the VT approach compared with the DVR approach. Nevertheless, our findings differ from the findings of studies conducted with chronic, treated samples, in which VT and DVR were both lower in SCZ, with large effect sizes (7), indicating that if there are SV2A deficits in early-course, untreated patients compared with control participants, they are likely to be less marked than in chronic, treated patients.
The patients in the previous studies had a mean illness duration of 17.4 years (7) and 17.3 years (8) and were mostly being treated with antipsychotic medication. In contrast, the mean illness duration of patients in the current study was 2.7 years, and none of our patients were taking antipsychotic medication. Thus, differences between the findings from the current study and earlier findings may be due to illness duration and/or antipsychotic exposure effects on SV2A levels (7,8). However, a rat study found no effect of antipsychotic treatment on SV2A markers (7), and the previous [11C]UCB-J studies in chronic SCZ found no relationship between antipsychotic exposure and [11C]UCB-J distribution volume in patients (7,8). Moreover, in this study, we found no association between drug-free interval duration and SV2A levels. Taken together, the evidence indicates that the difference between our current findings and earlier SV2A findings in SCZ is unlikely to be due to antipsychotic treatment effects. Instead, the differences could be due to differences in illness chronicity.
Another consideration is that patient ages differed between studies. In the earlier [11C]UCB-J studies in SCZ (7,8), the mean patient age was 41.5 and 40.5 years, respectively, whereas it was 26.5 years in the current study. Postmortem evaluations have indicated that dendritic spine density declines throughout the third decade of human life before stabilizing at around 30 years of age (21). It is possible that any illness effects on developmental synaptic pruning manifest only as small differences in SV2A levels during the third decade of life, in contrast to those identified during the fifth decade. However, the current study was only adequately powered to capture large effects. Moreover, age did not differ significantly between our groups, and findings remained essentially the same when age was added as a covariate to the analyses.
In the current study, mean PANSS scores were higher than in our previous study conducted with patients with chronic SCZ (7). Thus, the early-course group reported on here showed greater symptom severity than the chronic SCZ group that we reported on previously. The significant negative relationship between PANSS total scores and [11C]UCB-J VT in the hippocampus confirmed our hypothesis that SV2A levels are inversely associated with symptom severity, thereby extending preclinical and clinical evidence implicating hippocampal dysfunction in SCZ (14,22, 23, 24, 25), but contrasting with SV2A findings in chronic patients (7). Speculatively, these findings could suggest that SV2A levels are related to the disease process in early-course but not in chronic SCZ. Alternatively, the difference could be due to antipsychotic treatment in the chronic patients reducing symptom severity, hence the strength of the relationship with SV2A levels in the chronic SCZ study. Our finding of a link between SV2A levels and overall symptom severity in the hippocampus was not corrected for multiple comparisons, and we did not find this relationship in the FC or ACC or an association between [11C]UCB-J DVR and PANSS scores. Thus, additional studies are needed to test the link between SV2A measures and symptoms further.
Strengths and Limitations
Strengths of the current study include the fact that it is the first study to our knowledge to investigate SV2A levels early in the course of SCZ in untreated patients. While none of the patients included in this study were currently taking antipsychotic medication, most (n = 19) had previously done so. Thus, it is possible that prior antipsychotic treatment influenced our findings. This could explain why we found no significant differences between unmedicated patients and control participants in SV2A levels, whereas groups of medicated (7) and largely medicated patients (n = 10 of 13) (8) have shown large deficits in SV2A levels in diverse brain regions. However, the antipsychotics that patients had previously taken are not known to directly bind to SV2A (26). Moreover, previous studies have found no link between prior antipsychotic exposure and SV2A binding in the rat or human brain (7,8), and in this study, patients had a mean wash-out from antipsychotic treatment of 183 (minimum 41) days. Thus, it is unlikely that prior antipsychotic treatment explains our findings. Nevertheless, additional work is needed to test for the effects of antipsychotic exposure on SV2A levels.
It should be considered that the PET imaging has a transaxial spatial resolution of 4.6 mm and so is insensitive to alterations in regions smaller than this. Postmortem studies have identified lamina specificity in dendritic spine density alterations in SCZ compared with control subjects (27), particularly in cortical layer 3 (28, 29, 30). Thus, it remains possible that there are substructural group differences in SV2A levels that are obscured by PET resolution limits.
We used the CS, a white matter region largely devoid of SV2A, as a reference region to adjust for nonspecific binding when estimating [11C]UCB-J DVR (18). [11C]UCB-J DVR may reflect the SV2A-specific signal more closely than it reflects VT (31). However, white matter may serve suboptimally as a reference region given its distinct tissue composition from gray matter (31). Moreover, levetiracetam, an SV2A-selective drug, displaces a small amount of [11C]UCB-J in the CS, indicating low specific binding levels in this region (18). Group differences in CS-specific binding could have biased our findings, although we found no significant group differences in CS [11C]UCB-J VT. Thus, our DVR findings suggest a trend toward lower levels of SV2A-specific binding in patients with early-course, untreated SCZ than in control participants. However, our finding of lower temporal lobe DVR did not survive correction for multiple comparisons.
Finally, our study was powered to detect group differences of the magnitude previously detected in chronic SCZ, but a type II error is possible, and we were underpowered to detect more subtle differences. Notwithstanding this, inspection of the VT data (Figure 1) does not indicate a trend toward more subtle differences. Nevertheless, additional studies with early-course patients are warranted to confirm our findings.
Interpretation and Implications for the Synaptic Hypothesis of SCZ
[11C]UCB-J shows high specific binding to SV2A (32) and regional uptake consistent with prior anatomical knowledge regarding synaptic distribution (18). In gray matter, [11C]UCB-J shows displacement by levetiracetam, a drug specific to SV2A, indicating specific binding to SV2A (18). It also shows good test-retest reproducibility (18,19), indicating that [11C]UCB-J is a reliable SV2A-binding PET radioligand. SV2A is found ubiquitously in synaptic terminals throughout gray matter (33). Moreover, in the baboon brain, regional levels of SV2A are strongly positively correlated with those of synaptophysin (r > 0.95), which is often regarded as a gold standard ex vivo measure of synaptic density (18). It is possible that synaptic terminal density is lower in the absence of altered SV2A levels in early-course SCZ. However, this would require there to be greater SV2A levels per synaptic terminal in our patients, and when taken together with the evidence of lower SV2A levels in chronic patients, it would require this process to reverse at some point or for there to be very marked synaptic terminal density loss, which is not consistent with postmortem findings (3). We are unaware of any mechanism that could account for this. An alternative, more parsimonious explanation is that there are no, or subtle, synaptic terminal density deficits early in the course of SCZ and that these develop during the course of the illness, which would explain SV2A and postmortem synaptic density findings in patients with chronic illness (3,7,9,28), and be consistent with previous evidence for brain volume loss during the course of SCZ (34, 35, 36).
One mechanism that could account for synaptic changes in SCZ involves microglia (37,38). Microglia play a key role in synaptic pruning (39, 40, 41). Several lines of evidence indicate that this process may be abnormal in SCZ [reviewed in (42)], including findings of greater microglia-mediated synaptic engulfment in patient-derived neuronal preparations (43). Notwithstanding the caveats mentioned above, our current findings are not consistent with versions of the synaptic hypothesis that propose early developmental failure to form synapses and/or excess developmental synaptic pruning before illness onset (44,45). However, given that SV2A is a presynaptic marker, it remains unknown whether there are changes in postsynaptic density markers at illness onset in SCZ. Postsynaptic markers such as dendritic spine density and PSD-95 levels are significantly lower in SCZ in a meta-analysis of postmortem studies (28) and in numerous animal models of genetic (46,47) and environmental (48, 49, 50, 51, 52) risk factors for subjects with SCZ when compared to control subjects. The development of in vivo imaging probes specific to postsynaptic markers would be invaluable in assessing whether postsynaptic density changes emerge in patients during the course of SCZ. Moreover, the proposal that synaptic density changes occur during the course of illness remains speculative at this time, and it is important to consider alternative explanations. In particular, cohort effects, such as lower synaptic levels being specific to a subgroup of patients who are more likely to develop chronic illness, could account for differences between our current findings and previous findings with chronic patients. The generalizability of our sample to other settings should be considered. However, epidemiological and clinical trial data in first-episode samples have shown that ∼30% to 70% of patients discontinue antipsychotic treatment (53,54), indicating that our sample is not unusual in this respect. Longitudinal studies are needed to determine whether there are progressive SV2A reductions in SCZ over time.
Conclusions
Levels of [11C]UCB-J binding, a synaptic terminal marker, are not significantly different in patients with early-course SCZ compared with healthy volunteers, but we found preliminary evidence in exploratory analyses that there may be lower levels in the temporal lobe and some other brain regions and that greater symptom severity is associated with lower hippocampal synaptic marker levels. These findings are not consistent with our hypothesis that marked synaptic deficits are present early in the course of SCZ and indicate that the lower SV2A levels identified in patients with chronic SCZ may develop after illness onset, although longitudinal studies are warranted to confirm this.
Acknowledgments and Disclosures
For the purpose of open access, this paper has been published under a creative common license (CC-BY) to any accepted author manuscript version arising from this submission. This study was funded by Medical Research Council-UK (Grant Nos. MC_U120097115, MR/W005557/1, and MR/V013734/1 [to ODH]) and Wellcome Trust (Grant No. 094849/Z/10/Z [to ODH]) and by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. ECO acknowledges support from the National Institute for Health Research. The views expressed are those of the authors and not necessarily those of the National Health Service/National Institute for Health Research or the Department of Health.
We thank the patients and healthy volunteers who participated in this study and Yvonne Shearley, Rohini Akosa, Ryan Janisch, Daniela Ribeiro, Jim Anscombe, Dr. Sofia Pappa, Dr. William Hallett, Dr. Graham Searle, and Dr. Maja Ranger for their expert assistance.
EAR and RNG are employees of Invicro LLC. AM was an employee of Invicro LLC during the completion of much of this work. RNG is a consultant for AbbVie, Biogen, and Cerveau. TRM has received honoraria for speaking and chairing from Lundbeck, Janssen, and Astellas and received honoraria to participate in advisory boards organized by Angelini Pharmaceuticals. In the last 3 years, SJ has given nonpromotional educational talks for Lundbeck, Janssen, and Sunovion. ODH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Angellini, Autifony, Biogen, Boehringer Ingelheim, Eli Lilly, Heptares, Global Medical Education, Invicro, Jansenn, Lundbeck, Neurocrine, Otsuka, Sunovion, Recordati, Roche, and Viatris/Mylan. ODH has a patent for the use of dopaminergic imaging. The other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2023.05.022.
Contributor Information
Ellis Chika Onwordi, Email: e.onwordi@lms.mrc.ac.uk.
Oliver D. Howes, Email: oliver.howes@kcl.ac.uk.
Supplementary Material
References
- 1.Garey L.J., Ong W.Y., Patel T.S., Kanani M., Davis A., Mortimer A.M., et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glantz L.A., Lewis D.A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Osimo E.F., Beck K., Reis Marques T., Howes O.D. Synaptic loss in schizophrenia: A meta-analysis and systematic review of synaptic protein and mRNA measures. Mol Psychiatry. 2019;24:549–561. doi: 10.1038/s41380-018-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purcell S.M., Moran J.L., Fromer M., Ruderfer D., Solovieff N., Roussos P., et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fromer M., Pocklington A.J., Kavanagh D.H., Williams H.J., Dwyer S., Gormley P., et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekar A., Bialas A.R., de Rivera H., Davis A., Hammond T.R., Kamitaki N., et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onwordi E.C., Halff E.F., Whitehurst T., Mansur A., Cotel M.C., Wells L., et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 2020;11:246. doi: 10.1038/s41467-019-14122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radhakrishnan R., Skosnik P.D., Ranganathan M., Naganawa M., Toyonaga T., Finnema S., et al. In vivo evidence of lower synaptic vesicle density in schizophrenia. Mol Psychiatry. 2021;26:7690–7698. doi: 10.1038/s41380-021-01184-0. [DOI] [PubMed] [Google Scholar]
- 9.Onwordi E.C., Whitehurst T., Mansur A., Statton B., Berry A., Quinlan M., et al. The relationship between synaptic density marker SV2A, glutamate and N-acetyl aspartate levels in healthy volunteers and schizophrenia: A multimodal PET and magnetic resonance spectroscopy brain imaging study. Transl Psychiatry. 2021;11:393. doi: 10.1038/s41398-021-01515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 11.Mansur A., Rabiner E.A., Comley R.A., Lewis Y., Middleton L.T., Huiban M., et al. Characterization of 3 PET Tracers for Quantification of Mitochondrial and Synaptic Function in Healthy Human Brain: 18F-BCPP-EF, 11C-SA-4503, and 11C-UCB-J. J Nucl Med. 2020;61:96–103. doi: 10.2967/jnumed.119.228080. [DOI] [PubMed] [Google Scholar]
- 12.Grabner G., Janke A.L., Budge M.M., Smith D., Pruessner J., Collins D.L. In: Medical Image Computing and Computer- Assisted Intervention – MICCAI. Larsen R., Nielsen M., Sporring J., editors. Springer; Heidelberg: 2006. Symmetric atlasing and model based segmentation: An application to the hippocampus in older adults; pp. 58–66. [DOI] [PubMed] [Google Scholar]
- 13.Tziortzi A.C., Searle G.E., Tzimopoulou S., Salinas C., Beaver J.D., Jenkinson M., et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 14.Brugger S.P., Howes O.D. Heterogeneity and homogeneity of regional brain structure in schizophrenia: A meta-analysis. JAMA Psychiatry. 2017;74:1104–1111. doi: 10.1001/jamapsychiatry.2017.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neill A., Mechelli A., Bhattacharyya S. Dysconnectivity of large-scale functional networks in early psychosis: A meta-analysis. Schizophr Bull. 2019;45:579–590. doi: 10.1093/schbul/sby094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimol L.M., Hartberg C.B., Nesvåg R., Fennema-Notestine C., Hagler D.J., Jr., Pung C.J., et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 18.Finnema S.J., Nabulsi N.B., Eid T., Detyniecki K., Lin S.F., Chen M.K., et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96. doi: 10.1126/scitranslmed.aaf6667. [DOI] [PubMed] [Google Scholar]
- 19.Finnema S.J., Nabulsi N.B., Mercier J., Lin S.F., Chen M.K., Matuskey D., et al. Kinetic evaluation and test–retest reproducibility of [11C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab. 2018;38:2041–2052. doi: 10.1177/0271678X17724947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 21.Petanjek Z., Judaš M., Šimic G., Rasin M.R., Uylings H.B., Rakic P., Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieberman J.A., Girgis R.R., Brucato G., Moore H., Provenzano F., Kegeles L., et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: A selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23:1764–1772. doi: 10.1038/mp.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodge D.J., Grace A.A. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamminga C.A., Stan A.D., Wagner A.D. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 25.Roeske M.J., Lyu I., McHugo M., JU Blackford, Woodward N.D., Heckers S. Incomplete hippocampal inversion: A neurodevelopmental mechanism for hippocampal shape deformation in schizophrenia. Biol Psychiatry. 2022;92:314–322. doi: 10.1016/j.biopsych.2022.02.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaar S.J., Natesan S., McCutcheon R., Howes O.D. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172 doi: 10.1016/j.neuropharm.2019.107704. [DOI] [PubMed] [Google Scholar]
- 27.Kolluri N., Sun Z., Sampson A.R., Lewis D.A. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 28.Berdenis van Berlekom A., Muflihah C.H., Snijders G.J.L.J., MacGillavry H.D., Middeldorp J., Hol E.M., et al. Synapse pathology in schizophrenia: A meta-analysis of postsynaptic elements in postmortem brain studies. Schizophr Bull. 2020;46:374–386. doi: 10.1093/schbul/sbz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glausier J.R., Lewis D.A. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyer C.E., Shelton M.A., Sweet R.A. Dendritic spine alterations in schizophrenia. Neurosci Lett. 2015;601:46–53. doi: 10.1016/j.neulet.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossano S., Toyonaga T., Finnema S.J., Naganawa M., Lu Y., Nabulsi N., et al. Assessment of a white matter reference region for 11C-UCB-J PET quantification. J Cereb Blood Flow Metab. 2020;40:1890–1901. doi: 10.1177/0271678X19879230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nabulsi N.B., Mercier J., Holden D., Carré S., Najafzadeh S., Vandergeten M.C., et al. Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med. 2016;57:777–784. doi: 10.2967/jnumed.115.168179. [DOI] [PubMed] [Google Scholar]
- 33.Bajjalieh S.M., Frantz G.D., Weimann J.M., McConnell S.K., Scheller R.H. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14:5223–5235. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howes O.D., Cummings C., Chapman G.E., Shatalina E. Neuroimaging in schizophrenia: An overview of findings and their implications for synaptic changes. Neuropsychopharmacology. 2023;48:151–167. doi: 10.1038/s41386-022-01426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vita A., De Peri L., Deste G., Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: A meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2 doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziermans T.B., Schothorst P.F., Schnack H.G., Koolschijn P.C., Kahn R.S., van Engeland H., Durston S. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38:519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howes O.D., Onwordi E.C. The synaptic hypothesis of schizophrenia version III: A master mechanism. Mol Psychiatry. 2023;28:1843–1856. doi: 10.1038/s41380-023-02043-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howes O.D., McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: A reconceptualization. Transl Psychiatry. 2017;7 doi: 10.1038/tp.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calcia M.A., Bonsall D.R., Bloomfield P.S., Selvaraj S., Barichello T., Howes O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacol (Berl) 2016;233:1637–1650. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 41.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 42.Howes O.D., Shatalina E. Integrating the neurodevelopmental and dopamine hypotheses of schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry. 2022;92:501–513. doi: 10.1016/j.biopsych.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Sellgren C.M., Gracias J., Watmuff B., Biag J.D., Thanos J.M., Whittredge P.B., et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22:374–385. doi: 10.1038/s41593-018-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feinberg I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 45.Keshavan M.S., Anderson S., Pettegrew J.W. Is Schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 46.Papaleo F., Yang F., Paterson C., Palumbo S., Carr G.V., Wang Y., et al. Behavioral, neurophysiological, and synaptic impairment in a transgenic Neuregulin1 (NRG1-IV) murine schizophrenia model. J Neurosci. 2016;36:4859–4875. doi: 10.1523/JNEUROSCI.4632-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu W.S., Pesold C., Rodriguez M.A., Carboni G., Auta J., Lacor P., et al. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci USA. 2001;98:3477–3482. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirabella F., Desiato G., Mancinelli S., Fossati G., Rasile M., Morini R., et al. Prenatal interleukin 6 elevation increases glutamatergic synapse density and disrupts hippocampal connectivity in offspring. Immunity. 2021;54:2611–2631.e8. doi: 10.1016/j.immuni.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva-Gómez A.B., Rojas D., Juárez I., Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- 50.Comery T.A., Shah R., Greenough W.T. Differential rearing alters spine density on medium-sized spiny neurons in the rat corpus striatum: Evidence for association of morphological plasticity with early response gene expression. Neurobiol Learn Mem. 1995;63:217–219. doi: 10.1006/nlme.1995.1025. [DOI] [PubMed] [Google Scholar]
- 51.Colyn L., Venzala E., Marco S., Perez-Otaño I., Tordera R.M. Chronic social defeat stress induces sustained synaptic structural changes in the prefrontal cortex and amygdala. Behav Brain Res. 2019;373 doi: 10.1016/j.bbr.2019.112079. [DOI] [PubMed] [Google Scholar]
- 52.Li W.Y., Chang Y.C., Lee L.J., Lee L.J. Prenatal infection affects the neuronal architecture and cognitive function in adult mice. Dev Neurosci. 2014;36:359–370. doi: 10.1159/000362383. [DOI] [PubMed] [Google Scholar]
- 53.Kishi T., Ikuta T., Matsui Y., Inada K., Matsuda Y., Mishima K., Iwata N. Effect of discontinuation v. maintenance of antipsychotic medication on relapse rates in patients with remitted/stable first-episode psychosis: A meta-analysis. Psychol Med. 2019;49:772–779. doi: 10.1017/S0033291718001393. [DOI] [PubMed] [Google Scholar]
- 54.Morgan C., Lappin J., Heslin M., Donoghue K., Lomas B., Reininghaus U., et al. Reappraising the long-term course and outcome of psychotic disorders: The AESOP-10 study. Psychol Med. 2014;44:2713–2726. doi: 10.1017/S0033291714000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.