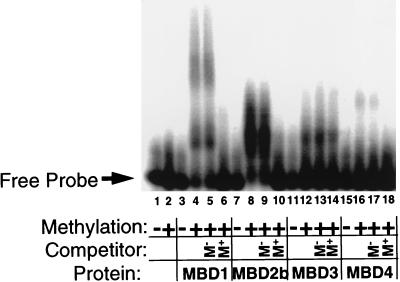
FIG. 4.
Binding of recombinant MBD proteins to methylated DNA in vitro. One hundred picograms of radiolabeled, double-stranded GAC (lanes 1, 3, 7, 11, and 15) or GAM12 (lanes 2, 4 to 6, 8 to 10, 12 to 14, and 16 to 18) DNA (Table 1) was incubated with recombinant MBD proteins in the presence (+) or absence (−) of 200 ng of unlabeled methylated or unmethylated competitor DNA and then electrophoresed through a 2% agarose gel. The location of the free probe is indicated, and protein-DNA interactions are evidenced as slower-migrating DNA. Approximate amounts of recombinant protein used were as follows: MBD1, 20 ng; MBD2b, 10 ng; MBD3, 100 ng; MBD4, 100 ng. All proteins form a slower-migrating complex with methylated probe but not with the unmethylated probe. The shifts of MBD1, MBD2b, and MBD4 are prevented by the presence of excess, unlabeled, methylated DNA during incubation (M+ Competitor), but not by the addition of excess unmethylated DNA (M− Competitor). The full-length MBD2 protein also produces a methyl-specific shift (data not shown). The shift produced by MBD3 is competed by neither methylated nor unmethylated DNA and is therefore considered to be nonspecific.