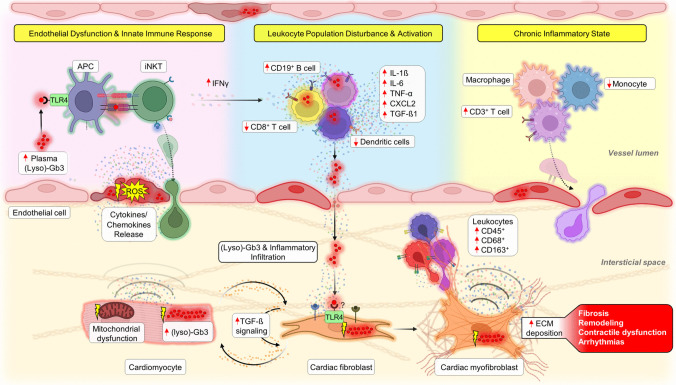
Fig. 1.
Integrated molecular pathways and cardiac implications in Fabry disease. Plasma globotriaosylceramide (Gb3) and globotriaosylsphingosine (lyso-Gb3), acting as both antigens and/or damage-associated molecular patterns (DAMPs) [34], bind to toll-like receptor 4 (TLR4) present on antigen-presenting cells (APCs). Upon this interaction, APCs enable the presentation of (lyso)-Gb3 to invariant natural killer T (iNKT) cells, thereby initiating pro-inflammatory signaling pathways, particularly leading to the release of interferon-gamma (IFN-γ; Pink field) [33, 99]. This interaction triggers a surge in pro-inflammatory mediators, predominantly released by peripheral blood mononuclear cells (PBMCs; blue field) [101•]. Concurrently, the shear stress and oxidative stress exerted on endothelial cells due to (lyso)-Gb3 deposition intensify local inflammatory cues, prompting iNKT cells to infiltrate the interstitium via the endothelial barrier [238]. This cascade amplifies cardiomyocyte stress, which is closely tied to mitochondrial dysfunction and (lyso)-Gb3 accumulation (orange field) [60•]. In this evolving inflammatory environment, macrophages, CD3+ T cells, and monocytes become prone to activation. These cells subsequently migrate and permeate the interstitium, further substantiating the chronic inflammatory response (yellow field) [27•]. Within the interstitial domain, (lyso)-Gb3 can also influence cardiac fibroblasts, promoting their activation. Herein, transforming growth factor-β (TGF-β) plays a critical role, driving myofibroblast differentiation and subsequent collagen deposition, accelerating the fibrotic progression and cardiac remodeling inherent in Fabry disease (orange field) [23–26, 27•]