Abstract
We have previously shown that a WD-40 repeat protein, TRIP-1, associates with the type II transforming growth factor β (TGF-β) receptor. In this report, we show that another WD-40 repeat protein, the Bα subunit of protein phosphatase 2A, associates with the cytoplasmic domain of type I TGF-β receptors. This association depends on the kinase activity of the type I receptor, is increased by coexpression of the type II receptor, which is known to phosphorylate and activate the type I receptor, and allows the type I receptor to phosphorylate Bα. Furthermore, Bα enhances the growth inhibition activity of TGF-β in a receptor-dependent manner. Because Bα has been characterized as a regulator of phosphatase 2A activity, our observations suggest possible functional interactions between the TGF-β receptor complex and the regulation of protein phosphatase 2A.
Mitogenic stimulation of cells by extracellular factors is often mediated by transmembrane tyrosine kinase receptors or receptors that associate with cytoplasmic tyrosine kinases. The signaling pathways generated by many of these receptors are well characterized (23). In contrast to the tyrosine kinase receptors, the receptor signaling pathways for transforming growth factor β (TGF-β) and the many TGF-β-related factors have only recently been characterized (12, 24). TGF-β and TGF-β-related factors are secreted proteins which mediate their activities through transmembrane serine/threonine kinase receptors. Ligand-induced activation of these receptors and signaling leads to potent growth inhibition and gene expression responses. Two type I and two type II receptors form the signaling TGF-β receptor complex at the cell surface, in which the type II receptors (TβRII) are constitutively active and autophosphorylated, and the type I receptors (TβRI) require phosphorylation by TβRII for activation (12, 24).
Several proteins have been shown to associate with TGF-β receptors. Smad2 and Smad3, which act as effectors of TGF-β signaling, can associate with the receptor complex and are phosphorylated by TβRI. Once dissociated, they are translocated as a complex with Smad4/DPC4 into the nucleus, where they function as transcriptional activators (11, 24, 33). Another receptor-associated protein is TRIP-1, which interacts with and is phosphorylated by TβRII (8) and contains five WD-40 repeats (40). WD-40 repeats are minimally conserved sequences of approximately 40 amino acids that typically end in tryptophan-aspartate (WD) and are thought to mediate protein-protein interactions (40). Since TRIP-1 is largely composed of WD-40 repeats, it is possible that other WD-40 repeat proteins may bind to serine/threonine kinase receptors. The association of WD-40 repeat proteins may then allow them to play a role in signaling by the serine/threonine kinase receptors. WD-40 repeats have been identified in a variety of proteins (40), including the Bα subunit of the serine/threonine protein phosphatase 2A (PP2A).
PP2A is one of the major, albeit poorly understood, serine/threonine phosphatases which regulates several processes, including signaling (43) and cell cycle progression (9, 44). PP2A exists as a dimeric core of a catalytic (C) and a structural (A) subunit or as a trimeric complex with a regulatory subunit (B), of which there are several forms. Bα and Bβ contain five WD-40 repeats (40), whereas B′/B56 (38) and B" (46) are structurally unrelated and lack WD-40 repeats (45, 46). Bα regulates the catalytic activity of PP2A, and this activity has been implicated in cell cycle control (21, 35). The differential interactions of these regulatory B subunits with the AC core enzyme suggest a complex pattern of regulation, which may explain the various functions of PP2A in growth control.
Because Bα has been implicated in cell cycle control (9, 21, 35) and has WD-40 repeats like TRIP-1 does, we analyzed the physical and functional interactions between Bα and TGF-β receptors. In this report, we demonstrate that the Bα regulatory subunit of PP2A interacts with the cytoplasmic domains of type I TGF-β receptors and is a direct target for their kinase activity. The growth inhibitory activity of Bα is regulated by TGF-β receptors and cooperates with the direct antiproliferative effect of the TGF-β receptors. Thus, the association of the WD-40 repeat Bα subunit of PP2A with serine/threonine kinase receptors results in a functional interaction of TGF-β receptor signaling with Bα and may be important for our understanding of how PP2A activity is regulated.
MATERIALS AND METHODS
In vitro translation and association with GST fusion proteins.
To generate 35S-labeled Bα or B′ in vitro, 2 μg of pRK7-Bα, i.e., the Bα cDNA subcloned in pRK7 (18), or pRK5-B′, i.e., the B′ cDNA in pRK5 (18) or pRK5-TRIP-1 (7), was used to transcribe the cDNAs from the SP6 promoter (Promega kit). The transcripts were translated and 35S labeled by using the TNT rabbit reticulocyte lysate kit (Promega) while being incubated at 30°C for 2 h. The translation mixture was then preadsorbed to glutathione S-transferase (GST) protein bound to glutathione-Sepharose beads in binding buffer (50 mM Tris-HCl [pH 7.5], 120 mM NaCl, 2 mM EDTA, 0.1% Nonidet P-40 [NP-40]) for 1 h at 4°C. The supernatant was then added to glutathione-Sepharose beads with 1 μg of purified GST protein or with 1 μg of the cytoplasmic domains of Tsk7L/R1 (amino acids 146 to 509), TβRI/R4 (amino acids 152 to 503), or TβRII (amino acids 194 to 567) fused to GST (GST-R1, GST-R4, or GST-TβRII) and incubated for 1 h at 4°C. Following adsorption, the glutathione beads with their adsorbed proteins were washed four times with binding buffer and specifically bound proteins were eluted twice with 50 μl of 50 mM Tris (pH 8.0)–5 mM reduced glutathione (42). Eluted proteins were diluted into loading buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The 35S-labeled bands were then detected by autoradiography.
Chemical cross-linking.
PP2A subunits and complexes, i.e. Bα, ABαC, and ABβC, were expressed in baculovirus-infected SF9 insect cells and purified as described previously (25). The His6-tagged cytoplasmic domains of the TGF-β receptors, i.e., TβRII (amino acids 194 to 567), TβRI/R4 (amino acids 152 to 503), and Tsk7L/R1 (amino acids 153 to 509), were also expressed in SF9 cells and affinity purified with Co2+-Sepharose beads and eluted from beads in 10 mM sodium phosphate (pH 8.0)–150 mM NaCl–100 mM imidazole. One microgram of Bα, ABαC, or ABβC was incubated with 1 μg of His6-tagged cytoplasmic domain of a receptor, and the protein interactions were stabilized by chemical cross-linking with 2 mM bismaleimidohexane (Pierce)–50 mM MOPS (morpholinepropanesulfonic acid; pH 7.0)–150 mM NaCl–1 mM EDTA for 90 min at 4°C. Protein samples were diluted into loading buffer and separated by SDS-PAGE. Following transfer of samples to nitrocellulose, Western blotting was performed by using anti-Bα antibody, and immunodetection was carried out by enhanced chemiluminescence (ECL) and autoradiography.
In vitro phosphorylation.
One hundred nanograms of His6-tagged cytoplasmic domain of Tsk7L/R1 was incubated alone, with 200 ng of Bα, or with 200 ng of ABαC in a kinase reaction mixture (27 mM HEPES [pH 7.4], 4 mM MnCl2, 40 mM nitrophenylphosphate, 10 μCi of [γ-32P]ATP per nmol). Reaction mixtures were incubated for 30 min at 30°C, proteins were separated by SDS-PAGE, and 32P-labeled bands were visualized by autoradiography.
In vivo association of Bα with the receptor cytoplasmic domains.
COS-1 cells were transfected by using Lipofectamine (Gibco-BRL) with expression plasmids while the total amount of plasmid DNA was kept at 15 μg. To express Bα, we used 10 μg of pCMV5-Bα, i.e., pCMV5 (2) containing the coding sequence for Bα with a C-terminal FLAG tag, whereas the receptor cytoplasmic domains were expressed from 5 μg of pRK5 encoding His6-tagged kinase-active or -inactive cytoplasmic domains of Tsk7L/R1 (amino acids 153 to 509), TβRI/R4 (amino acids 152 to 503), or TβRII (amino acids 194 to 567). To determine the effect of full-length TβRII on the association of Bα with the cytoplasmic domain of R4, 0, 1, or 10 μg of pRK5-TβRII (RII) was cotransfected with TβRI/R4 and pCMV5-Bα as described above, except that total DNA was kept at 25 μg. At 24 h after transfection, the cells were washed and labeled with 100 μCi of [35S]Met-Cys in Dulbecco modified Eagle medium (DMEM)–10% fetal bovine serum (FBS) for 16 h. The cells were washed with phosphate-buffered saline (PBS) and lysed with 10 mM Na phosphate (pH 8.0)–150 mM NaCl–0.1% NP-40, 1 mM phenylmethylsulfonyl fluoride (PMSF)–1 mM benzamidine. Cleared lysates were then incubated with Co2+-Sepharose beads for 1 h at 4°C, and the beads were washed with 10 mM Na phosphate (pH 8.0)–150 mM NaCl–25 mM imidazole (Sigma). Specifically bound proteins, i.e. His6-tagged cytoplasmic domains with associated proteins, were eluted with 10 mM Na phosphate (pH 8.0)–150 mM NaCl–100 mM imidazole. The proteins were separated by SDS-PAGE and transferred to nitrocellulose, and receptor-associated Bα was identified by Western blotting with anti-Bα antibody, ECL, and autoradiography.
Cell surface biotinylation and coimmunoprecipitation of receptors with Bα.
COS-1 cells in 10-cm-diameter plates were transfected by using Lipofectamine with 16 μg of plasmid DNA. To express Bα, we used 8 μg of pCMV5-Bα, i.e., pCMV-5 containing the coding sequence of Bα with a C-terminal FLAG tag, whereas full-length type I receptors were expressed from 8 μg of pRK5 encoding C-terminally Myc-tagged Tsk7L/R1 or TβRI/R4. At 36 h after transfection, the cells were washed with PBS and incubated with 1 mg of normal human serum-LC-biotin at 4°C for 45 min. The cells were then washed and incubated for 10 min with 50 mM glycine in PBS–0.5 mM MgCl2 (pH 7.8). The cells were lysed with 50 mM HEPES (pH 7.5)–1% NP-40, and cleared lysates were incubated with anti-FLAG antibody and protein A-Sepharose beads. The beads were then washed with a series of buffers, starting with 12.5 mM K phosphate (pH 7.4)–0.6 M NaCl, and then 10 mM Tris (pH 8.3)–0.1% SDS–0.05% NP-40–0.3 M NaCl, followed by 12.5 mM K phosphate (pH 7.5)–0.6 M NaCl and 12.5 mM K phosphate (pH 7.5)–0.3 M NaCl, and finally 20 mM Tris (pH 8.3). Bα and coprecipitating proteins were eluted with gel sample buffer, analyzed by SDS-PAGE, and transferred to nitrocellulose, and the biotinylated bands were identified by using horseradish peroxidase-conjugated streptavidin and ECL.
In vivo phosphorylation of N-terminally truncated Bα.
COS-1 cells were cotransfected by using Lipofectamine with pRK5-Myc-Bα#50-447, encoding Bα lacking N-terminal amino acids 1 to 49, with or without pRK5-TβRII (TβRII) and pRK5-R4 (R4). At 36 h after transfection, the cells were labeled with 1 mCi of [32P]orthophosphate per ml for 12 h, incubated with 1 μM okadaic acid for 1 h, and then stimulated for 30 min with 400 pM TGF-β. The cells were washed and lysed at 4°C in 27 mM HEPES (pH 7.4)–150 mM NaCl–0.5 mM EGTA–0.1% Triton X-100–1 μM okadaic acid–40 mM nitrophenylphosphate–100 μM orthovanadate–1 mM PMSF–1 mM benzamidine. 32P-labeled Bα#50-447 was immunoprecipitated with anti-Myc 9E10 antibody and detected after SDS-PAGE and autoradiography of 32P-labeled bands.
In vivo TGF-β receptor phosphorylation.
L17 cells, a highly transfectable Mv1Lu mutant cell line lacking functional TβRI/R4 (48), were cotransfected by using Lipofectamine (Gibco-BRL) with 2 μg of the Bα expression plasmid pRK7-Bα, or the parental pRK7 plasmid, and 1 μg of pRK5-TβRII-Flag (TβRII) and pRK5-R4-Flag (R4). At 36 h after transfection, the cells were washed and labeled with 1 mCi of [32P]orthophosphate in DMEM without phosphate–10% FBS for 4 h. The cells were stimulated for 30 min with 400 pM TGF-β and washed and lysed at 4°C in 27 mM HEPES (pH 7.4)–150 mM NaCl–0.5 mM EGTA–0.1% Triton X-100–40 mM nitrophenylphosphate–100 μM orthovanadate–1 mM PMSF–1 mM benzamidine. Receptors were immunoprecipitated with anti-FLAG M2 antibody, purified proteins were separated by SDS-PAGE, and receptors were detected by autoradiography of 32P-labeled bands.
PP2A assay.
The expression plasmid pRK7-Bα or pRK7 were cotransfected with pHook2/neo (Invitrogen) into HaCaT cells, and pools of stably transfected cells were selected by using 750 μg of G418 (Sigma) per ml. The cells were then plated in six-well dishes and grown to 70% confluence in DMEM containing 10% FBS. After incubation with or without 400 pM TGF-β for 10 min, the cells were placed on ice, washed, and lysed. The lysates were diluted to the same protein concentration, and equal aliquots were assayed for phosphatase activity for 10 min at 30°C in 20 mM MOPS (pH 7.0)–0.5-mg/ml BSA–0.5 mM dithiothreitol (DTT)–1-mg/ml [32P]phosphorylase A (prepared by using the Gibco BRL phosphatase kit) in the presence or absence of 1 nM okadaic acid (Gibco-BRL). Reactions were terminated by adding 15% trichloroacetic acid and 2.5 mg of BSA per ml. Proteins were precipitated for 10 min at 4°C and pelleted by centrifugation for 5 min. 32P released into the supernatant was measured by liquid scintillation counting. PP2A activity was defined as the phosphorylase A phosphatase activity inhibitable by 1 nM okadaic acid (10).
PAI-1- and cyclin A-luciferase assays in HaCaT cells.
HaCaT cells (16) were grown to 40% confluence in six-well dishes. An 0.8-μg amount of pRK7-Bα or pRK7 was cotransfected with Lipofectamine with 0.4 μg of pRKβgal and either 0.4 μg of p3TP-lux, for PAI-1-luciferase assays (47), or 0.4 μg of pCal2, for cyclin A-luciferase activity (14), and incubated at 37°C for 18 h. In parallel experiments, 0.8 μg of Bα or 0.4 μg of pRK5-Smad3 and 0.4 μg of pRK5-Smad4 were transfected with 0.5 μg of pCal2 and 0.5 μg of pSVβgal and incubated at 37°C for 18 h. The medium was then replaced with DMEM–0.2% FBS with or without 100 pM TGF-β and incubated for 24 h. Cleared lysates were prepared and assayed for luciferase (by using Promega’s assay kit and a Monolight 2010 luminometer from Analytical Luminescence Laboratory) and β-galactosidase (Tropix kit) activities. The luciferase activity was normalized against the β-galactosidase activity as a measure of transfection efficiency.
PAI-1 protein production and DNA synthesis in transfected HaCaT cells, overexpressing Bα.
To measure PAI-1 protein production, pools of HaCaT cells, transfected with pRK7 or pRK7-Bα, were grown to 50% confluence in six-well dishes in DMEM–10% FBS. The cells were washed with PBS and incubated in a mixture of DMEM without Met-Cys and 25 μCi of [35S]Met per ml, with or without 100 pM TGF-β, for 2 h at 37°C. The cells were washed, and extracellular matrix proteins were purified as described previously (22). 35S-labeled proteins, including PAI-1, were separated by SDS-PAGE and detected by autoradiography. To measure DNA synthesis, HaCaT cells transfected with pRK7 or pRK7-Bα were plated in 24-well dishes and grown to 50% confluence. The cells were washed with PBS and incubated with 0.2% FBS with or without 100 pM TGF-β for 20 h at 37°C. A 4-μCi/ml concentration of [3H]thymidine was added to the medium for 4 h, and the cells were washed and trypsinized. Trypsinized cells were collected on Whatman GF/C filters in a filtration apparatus, washed with PBS–10% trichloroacetic acid, and dried, and the radioactivity on the filters was measured by scintillation counting.
Cyclin A-luciferase assays in SW480.7 cells.
SW480.7 cells (17, 49) were grown to 60% confluence in six-well dishes. An 0.8-μg amount of pCMV5-Bα or pRK7 was cotransfected by using Lipofectamine with 0.4 μg of pRK5-Smad3 and 0.4 μg of pRK5-Smad4 or pRK5 (as control), and 0.4 μg of pCal2 for cyclin A-luciferase activity (14). Transfected cells were incubated at 37°C for 18 h. Media were then replaced with DMEM–0.2% FBS with or without 100 pM TGF-β and incubated for 24 h. Cleared lysates were prepared and assayed for luciferase and β-galactosidase activities. The luciferase activity was normalized against the β-galactosidase activity as a measure of transfection efficiency.
Cyclin A-luciferase assay in Mv1Lu cells.
Confluent Mv1Lu cells trypsinized from a 100-mm-diameter plate were electroporated with 45 μg of plasmid DNA in DMEM by using 0.4-cm-gap cuvettes (Bio-Rad) at 960 μF and 350 V. The electroporated DNA contained 25 μg of pRK7-Bα or pRK7, 5 μg of pRK5-TβRIDN (DN R4), which drives the expression of cytoplasmically truncated TβRI/R4, 5 μg of pCal2, 5 μg of pRKβgal, and 5 μg of pBluescript. Electroporated cells were allowed to recover for 4 h at 37°C in DMEM–10% FBS and then washed with PBS and incubated in the presence or absence of 100 pM TGF-β in DMEM–0.2% FBS. After 48 h, cleared lysates were prepared and assayed for luciferase and β-galactosidase activities.
Finally, cyclin A-luciferase activity was also assayed in transfected DR26 and R1B cells, mutant Mv1Lu cells that lack functional TβRII and TβRI, respectively (29, 30). Confluent cells were trypsinized from a 100-mm-diameter plate and electroporated with 55 μg of plasmid DNA. The electroporated DNA contained 25 μg of pRK7-Bα or pRK7, 15 μg of pRK5-TβRI (RI) for R1B cells or 15 μg of pRK5-TβRII (RII) for DR26 cells, and 5 μg of pCal2, 5 μg of pSVβgal, and 5 μg of pBluescript. Electroporated cells were allowed to recover and processed as described for Mv1Lu cells.
RESULTS
In vitro association of the Bα subunit of PP2A with type I TGF-β receptors.
To evaluate the association of the WD-40 repeat protein Bα with serine/threonine kinase receptors, we first assessed its ability to directly interact with their cytoplasmic domains in vitro. Three receptors were tested for their ability to associate with Bα: the type II TGF-β receptor (TβRII) (31) and two type I receptors, TβRI/R4 (15), which mediates growth inhibition and gene induction by TGF-β in various cell types (3, 15), and Tsk7L/R1 (13), which is involved in TGF-β-mediated transdifferentiation of NMuMG cells (39) and responds to bone morphogenetic proteins when coexpressed with the bone morphogenetic protein type II receptor (32). 35S-labeled, in vitro-translated Bα interacted with the cytoplasmic domains of the two type I receptors fused to (GST), but not with GST alone, and only minimally with the TβRII cytoplasmic domain (Fig. 1A). In contrast, TRIP-1, which like Bα has five WD-40 repeats and associates with the TβRII receptor (8), did not interact with the type I receptor cytoplasmic domain (Fig. 1B), consistent with our previous results (8). We therefore conclude that Bα specifically and directly associates with the type I receptor cytoplasmic domain in vitro.
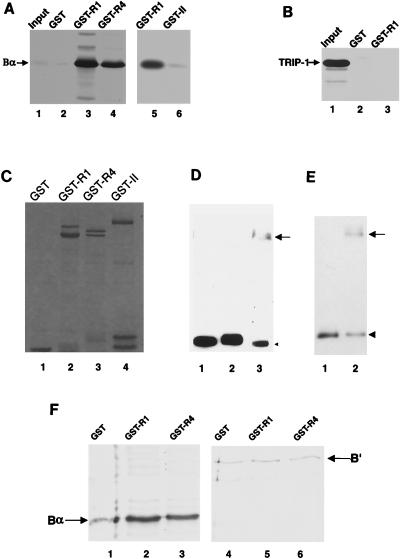
FIG. 1.
In vitro association of Bα with type I receptors. (A) Association of in vitro-translated Bα with GST-receptor fusion proteins. In vitro-translated 35S-labeled Bα was incubated with glutathione-Sepharose-coupled GST (lane 2) or GST fused to the cytoplasmic domains of Tsk7L/R1 (lanes 3 and 5), TβRI/R4 (lane 4), or TβRII (lane 6). Bound proteins were eluted and separated by SDS-PAGE. Lane 1 shows 1/50 of the input 35S-labeled Bα used in each of the GST adsorption experiments. (B) Lack of interaction of TRIP-1 with the cytoplasmic domain of a type I receptor fused to GST (GST-R1) or with GST itself. (C) Gel electrophoretic analysis of the purified GST fusion experiments used in panel A and other experiments. Whereas the GST protein itself corresponded to a single band on the gel, the fusion proteins ran as several bands, the largest of which corresponded to the full-size proteins, and the smaller bands are presumably degradation products. (D) Covalent cross-linking of Bα in the ABαC complex with the cytoplasmic domain of Tsk7L/R1. Purified ABαC complex was incubated without cross-linking (lane 1) or was chemically cross-linked in the absence (lane 2) or presence (lane 3) of purified cytoplasmic domain of Tsk7L/R1. Cross-linked proteins were separated by SDS-PAGE and immunoblotted with anti-Bα antibody. The arrow points to the cross-linked complex of Bα with Tsk7L/R1 which is detected as an anti-Bα immunoreactive band. The arrowhead points to noncomplexed Bα. (E) Covalent cross-linking of Bβ in the ABβC complex with the cytoplasmic domain of Tsk7L/R1. Purified ABβC was chemically cross-linked in the absence (lane 1) or presence (lane 2) of the His6-tagged cytoplasmic domain Tsk7L/R1. The arrow points to the cross-linked complex of Bβ with Tsk7L/R1 which is detected as the anti-Bβ immunoreactive band, and the arrowhead points to noncomplexed Bβ. (F) In vitro-translated B′ does not bind GST-receptor fusion proteins. In vitro-translated 35S-labeled Bα (lanes 1 to 3) and B′ (lanes 4 to 6) were tested for their association with GST (lanes 1 and 4) or GST fusion proteins with the cytoplasmic domains of Tsk7L/R1 (lanes 2 and 5) or TβRI/R4 (lanes 3 and 6) as described for panel A.
Since Bα interacts with the heteromeric AC core enzyme of PP2A, we next incubated purified ABαC complex with the His6-tagged cytoplasmic domain of Tsk7L/R1 to determine whether Bα in complex with AC could associate with type I receptors either in the monomeric form or as part of an intact complex. We then stabilized the interaction by chemical cross-linking, a method used to detect interactions between the three subunits of the PP2A complex (25). Bα interacted with the cytoplasmic domain of Tsk7L/R1 under these conditions, thus resulting in a 100-kDa cross-linked band with immunoreactivity for anti-Bα (Fig. 1D) and anti-Tsk7L/R1 (data not shown). The use of purified ABαC in this experiment indicates that when provided in a complex with PP2A, Bα is able to directly associate with Tsk7L/R1. Under these conditions, the ABαC complex was also formed but not resolved on the gel (data not shown). Considering the large size and low efficiency of cross-linking, it was difficult to assess whether a complex of ABαC with Tsk7L/R1 was formed.
Besides Bα (21), the AC subunits of PP2A can also interact with other B subunits (25). One of these, Bβ, also contains WD-40 repeats yet has a highly divergent N-terminal sequence (34). In contrast, another B subunit, B′, lacks WD-40 repeats and is unrelated (46). Like Bα, Bβ was able to directly associate with the cytoplasmic domain of Tsk7L/R1, as determined by chemical cross-linking (Fig. 1E). In contrast, 35S-labeled B′ did not interact with the cytoplasmic domains of the receptors (Fig. 1F) and B′ in purified AB′C complex also did not detectably interact (data not shown), suggesting that WD-40 repeats may be involved in the interaction with type I receptors.
Type I receptor kinase-dependent association and phosphorylation of Bα.
Type I receptors, like type II receptors, are serine/threonine kinases. Since the WD-40 repeat protein TRIP-1 interacts with higher affinity with the kinase-active than with the kinase-inactive type II receptor (8), we examined whether association of Bα with the type I receptor also depended on its kinase activity. As shown in Fig. 2A, Bα associated with the kinase-active cytoplasmic domain (lane 4) but not with a kinase-inactive point mutant (lane 3) and was detected as a 100-kDa, cross-linked anti-Bα immunoreactive band.
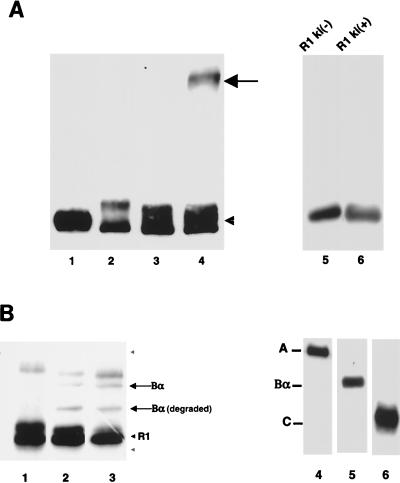
FIG. 2.
Interaction of Bα with the type I receptor cytoplasmic domain depends on its kinase activity and allows phosphorylation of Bα by the receptor kinase. (A) Bα interacts with kinase-active [ki(+)], not with kinase-inactive [ki(−)], Tsk7L/R1. Bα in the absence of the cytoplasmic domain of the receptor without (lane 1) or after (lane 2) chemical cross-linking is shown. Bα incubated with the kinase-inactive (lane 3) or kinase-active (lane 4) cytoplasmic domain of the receptor and stabilized by chemical cross-linking is also shown. The arrow points to the cross-linked complex of Bα with Tsk7L/R1 which is detected as the anti-Bα immunoreactive band, and the arrowhead points to noncomplexed Bα. Lanes 5 and 6 show that equal amounts of the kinase-negative or -positive cytoplasmic domains of Tsk7L/R1, purified as His6-tagged proteins, were used in lanes 3 and 4. (B) Tsk7L/R1 phosphorylates Bα. The purified cytoplasmic domain of Tsk7L/R1 was incubated alone (lane 1), with Bα (lane 2), or with ABαC (lane 3) in a kinase reaction in the presence of [γ-32P]ATP. Proteins were separated by SDS-PAGE, and 32P-phosphorylated substrates were visualized by autoradiography. Besides Bα, a degradation product of Bα [Bα (degraded)] was also phosphorylated. The phosphorylated band above the Bα band is a protein that copurified with the receptor cytoplasmic domain. The gray arrowheads denote the positions of the A (upper) and C (lower) subunits, which were not phosphorylated. Lanes 4 to 6 show Western blots of purified and electrophoretically separated ABαC (26), which was used in lane 3, with antibodies specific for A, Bα, or C, respectively.
We next assessed whether Bα is a substrate for the kinase activity of Tsk7L/R1. The His6-tagged cytoplasmic domain of Tsk7L/R1, purified from baculovirus-infected cells, was incubated with purified Bα in a kinase assay with [γ-32P]ATP, either alone or in the presence of purified ABαC complex, in which all three protein components are present in an equimolar ratio (25). As shown in Fig. 2B, the type I receptor (Tsk7L/R1) phosphorylated both purified Bα (lane 2) and Bα, but not A and C, in the ABαC complex (lane 3). The lack of phosphorylation of A and C in these assays illustrated the specificity of Bα as a kinase substrate.
Interaction of Bα with type I receptors in vivo.
To determine whether Bα associates with the type I receptor in vivo, we coexpressed Bα with the type I receptors and assessed their interaction by using coimmunoprecipitation and Western blot analyses. To distinguish between Bα, the type I receptors, and the antibody heavy chain, which all migrate with the same mobility in SDS-PAGE, we coexpressed Bα with the cytoplasmic domains of the receptors preceded with an N-terminal methionine and a His6 sequence. Purification of the receptor cytoplasmic domains by adsorption to Co2+-Sepharose thus allowed copurification of receptor-associated Bα. Subsequent Western blot analyses using anti-Bα antiserum showed that Bα associated with the cytoplasmic domains of the TβRI and Tsk7L type I receptors (Fig. 3A, lanes 3 and 5) but did not interact with the kinase-inactive mutants of these receptors (Fig. 3A, lanes 2 and 4) nor with the type II receptor cytoplasmic domain (Fig. 3A, lanes 6 and 7). The A and C proteins were not detectable by using Western blot analyses or assays for phosphatase activity (data not shown), suggesting that receptor-bound Bα may not interact with the AC core enzyme or that low levels of associated AC may not be detectable. The low endogenous receptor levels and limitations in quality of the antibodies made detection of the association of endogenous Bα and type I receptors technically not feasible.
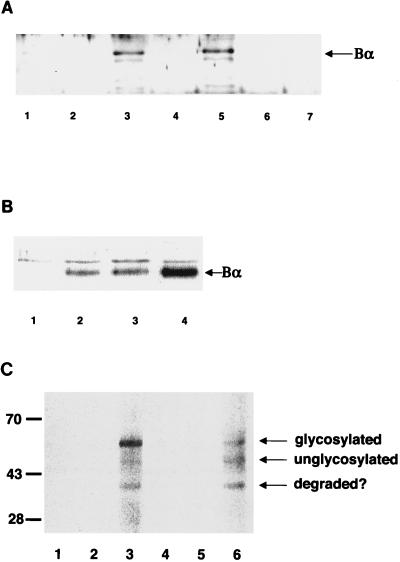
FIG. 3.
In vivo association of Bα with type I receptors. (A) In vivo association of Bα with type I receptors. Bα was expressed alone (lane 1) or coexpressed with His6-tagged kinase-inactive (lanes 2, 4, and 6) or kinase-active (lanes 3, 5, and 7) cytoplasmic domains of the type I receptor Tsk7L/R1 (lanes 2 and 3) or TβRI/R4 (lanes 4 and 5) or the type II receptor TβRII (lanes 6 and 7) in transfected COS-1 cells. His6-tagged cytoplasmic domains and associated proteins were purified on Co2+-Sepharose beads and, following elution, separated by SDS-PAGE, and Bα was detected by immunoblotting with anti-Bα antibody. (B) TβRII coexpression enhances the association of Bα with TβRI. Bα was expressed alone (lane 1) or was coexpressed with the His6-tagged cytoplasmic domain of TβRI/R4 (lanes 2 to 4) in transfected COS-1 cells, in the absence (lane 2) or presence (lanes 3 and 4) of TβRII (1 [lane 3] or 10 [lane 4] μg of transfected plasmid DNA). As in panel A, the proteins associated with TβRI purified on Co2+-Sepharose beads were separated by SDS-PAGE and associated Bα was detected by immunoblotting. (C) Interaction of Bα with full-size type I receptor at the cell surface. The full-size type I receptor Tsk7L/R1 (lanes 2 and 3) or TβRI/R4 (lanes 5 and 6) was expressed in COS-1 cells in the presence (lanes 3 and 6) or absence (lanes 2 and 5) of Bα in transfected cells. The corresponding cytoplasmically truncated Tsk7L/R1 (lane 1) or TβRI/R4 (lane 4) was also coexpressed with Bα. Cell surface proteins were labeled by surface biotinylation of intact cells, and immunoprecipitations were carried out by using anti-Bα antibody, followed by visualization of coprecipitated, biotinylated proteins. The full-size glycosylated and unglycosylated type I receptor and an often-observed degradation product (degraded?) are indicated.
Although the type I receptors have an inherent kinase activity, their phosphorylation and kinase activity are greatly enhanced following ligand-induced phosphorylation by the type II receptor kinase (48). Additionally, coexpression of the type II TGF-β receptor cytoplasmic domain resulted in direct phosphorylation of the interacting type I receptor (7, 14). We therefore coexpressed the type II receptor with TβRI/R4 to see if it influenced the level of Bα associated with the type I receptor. As shown in Fig. 3B, increasing TβRII expression strongly enhanced the level of Bα associated with TβRI/R4, suggesting that Bα associates with the activated receptor. Auto- and transphosphorylation of the type I receptor, therefore, is likely to increase the affinity of the receptor for Bα.
Finally, we also evaluated whether Bα could interact with full-length type I receptors at the cell surface. Thus, we expressed Bα and type I receptor TβRI/R4 or Tsk7L/R1 and labeled the cell surface proteins by biotinylation. As shown in Fig. 3C, immunoprecipitation of Bα resulted in coprecipitation of cell surface type I receptors. As expected, cytoplasmically truncated type I receptors did not associate with Bα. This illustrates that Bα can associate with the cytoplasmic domains of full-length type I receptors at the cell surface.
In vivo phosphorylation of N-terminally truncated Bα.
Whereas the phosphorylation of Bα was readily demonstrated in vitro, it was much harder to define conditions that convincingly showed receptor-dependent phosphorylation of Bα in vivo. In the absence of okadaic acid, phosphorylation of Bα was not detected with or without overexpression of TGF-β receptors. In contrast, in the presence of 1 μM okadaic acid, Bα was already highly phosphorylated in the absence of exogenous TGF-β and this high level was only minimally enhanced by TGF-β (data not shown). The lack of convincing evidence for Bα phosphorylation by TGF-β receptors under our conditions may be due to the endogenous autocrine activation of the TGF-β receptors, and the very transient nature of the phosphorylation may be due to rapid dephosphorylation of Bα by the core PP2A enzyme. We therefore analyzed the ability of the receptors to phosphorylate a mutant of Bα which lacks its N-terminal 49 amino acids. This small truncation abolished the interaction of Bα with the A and C subunits of PP2A (46a). As shown in Fig. 4, this Bα mutant is phosphorylated only when coexpressed with TGF-β receptors. This observation demonstrates the ability of TGF-β receptors to phosphorylate Bα in vivo. Our inability to convincingly demonstrate full-size Bα phosphorylation by the receptor may be due to its rapid association with and dephosphorylation by the PP2A core enzyme.
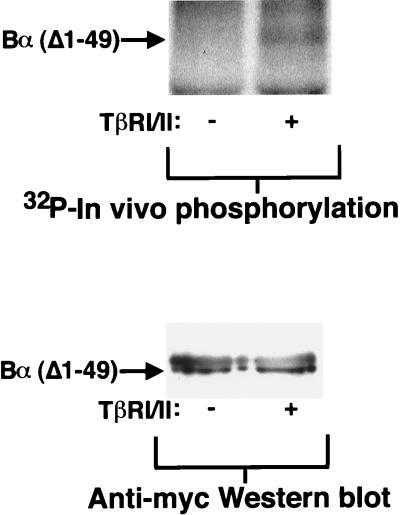
FIG. 4.
N-terminally truncated Bα is phosphorylated upon coexpression of TβRI and TβRII. Myc-epitope-tagged Bα, lacking amino acids 1 to 49, was coexpressed in the presence (+) or absence (−) of TβRI/R4 and TβRII (TβRI/II) in transfected COS cells. The in vivo phosphorylation of this deletion mutant of Bα was assessed by in vivo 32P labeling followed by immunoprecipitation with anti-Myc antibody 9E10 and detection by SDS-PAGE and autoradiography (upper lanes). The lower lanes illustrate equal expression levels of the Myc-tagged Bα mutant as assessed by anti-Myc Western blotting of anti-Myc immunoprecipitated protein. Left lanes, without TβRI/TβRII expression; right lanes, with TβRI/TβRII expression.
Bα expression does not affect receptor phosphorylation.
Since the Bα subunit can associate with the type I receptors and also can regulate PP2A activity through its association with the AC core enzyme (25), it is possible that Bα recruits the phosphatase to the activated receptor complex, which could then alter the phosphorylation state of the receptors. To test this, we coexpressed TβRI/R4 and TβRII with or without Bα and analyzed the in vivo 32P phosphorylation levels of the receptors after TGF-β stimulation. The level of phosphorylation of TβRI/R4 or TβRII, however, was not detectably altered with increased Bα expression (Fig. 5). In addition, no time-dependent alteration in the level of receptor phosphorylation was observed following TGF-β stimulation (data not shown). Therefore, association of Bα with the receptors does not result in detectable overall changes in receptor phosphorylation, although differential phosphorylation of individual amino acids cannot be excluded.
FIG. 5.
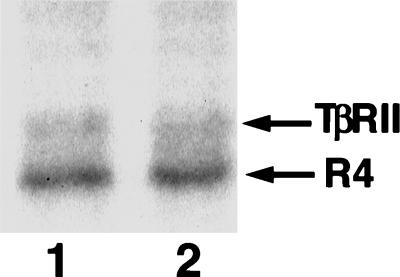
Increased Bα expression does not alter the overall phosphorylation state of TGF-β receptors. FLAG-tagged type II receptors (TβRII) and type I receptor TβRI/R4 (R4) were coexpressed with Bα in transfected L17 cells. The in vivo phosphorylation of the receptors was assessed by in vivo 32P labeling followed by immunoprecipitation with FLAG antibody and detection by SDS-PAGE and autoradiography. Lane 1, without increased Bα expression; lane 2, with increased Bα expression.
Increased expression of Bα regulates TGF-β responsiveness.
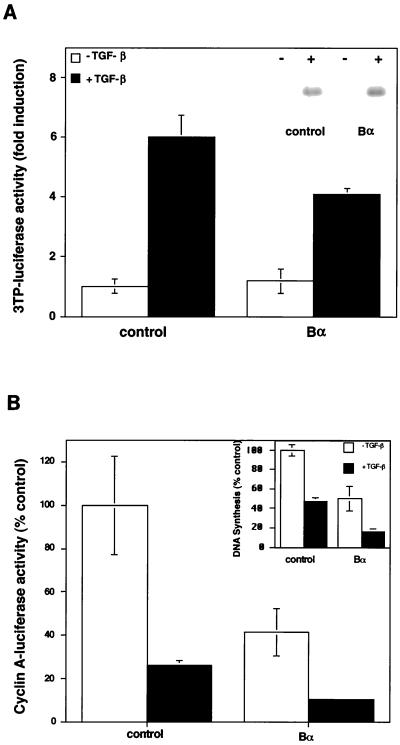
The lack of detectable effect of Bα on receptor phosphorylation does not preclude that Bα might regulate downstream TGF-β signaling. We therefore tested the effect of Bα on two TGF-β responses in HaCaT cells, a cell line which is highly sensitive to the antiproliferative effect of TGF-β (16). In one set of assays, TGF-β-induced gene expression was assessed by measuring luciferase expression from the PAI-1 promoter (27) or the 3TP promoter (47) or by measuring PAI-1 protein expression (22) in stably transfected cells. TGF-β greatly induced transcription from the 3TP promoter (Fig. 6A) or the PAI-1 promoter (data not shown). Increased Bα expression resulted in a small but reproducible decrease in the TGF-β response without affecting the basal level of expression from the PAI-1 promoter (Fig. 6A). This decrease was also apparent in Mv1Lu epithelial cells (data not shown). Expression of the PAI-1 protein in HaCaT cells, stably transfected with a Bα expression plasmid, was, however, not decreased when compared to that of control transfected cells (Fig. 6A, inset). The discrepancy between these results may be due to measuring PAI-1 transcription versus protein levels.
FIG. 6.
Effect of Bα on TGF-β responsiveness. (A) Effect of Bα on luciferase expression from the 3TP promoter. The Bα expression plasmid pRK7-Bα or the control plasmid pRK7 was cotransfected with p3TP-lux in HaCaT cells. Luciferase expression was measured in the absence or presence of TGF-β. The inset shows that PAI-1 protein levels are not altered by Bα in stably transfected cell lines. 35S-labeled PAI-1 protein secreted from HaCaT cells, stably transfected with pRK7 (control) or pRK7-Bα in the absence (−) or presence (+) of 100 pM TGF-β, was used. (B) Bα enhances the inhibition of luciferase activity by TGF-β from the cyclin A promoter. HaCaT cells were cotransfected with the pCal2 luciferase reporter plasmid and the control plasmid pRK7 or the Bα expression plasmid pRK7-Bα. Luciferase expression from the cyclin A promoter, a measure of cell proliferation, was measured in the absence or presence of TGF-β. the inset shows that Bα increases the inhibition of DNA synthesis by TGF-β in stably transfected cells. DNA synthesis, measured by [3H]thymidine incorporation in HaCaT cells, stably transfected with pRK7 (control) or pRK7-Bα in the absence (−) or presence (+) of 100 pM TGF-β, is shown. Data are presented relative to those of untreated, control transfected cells. Standard deviations were based on triplicate measurements.
We also measured the effect of Bα on TGF-β-induced growth inhibition in HaCaT cells (16) and Mv1Lu cells (29) by using a reporter assay in which decreased luciferase expression from the cyclin A promoter correlates with growth inhibition (14). Thus, treatment with TGF-β resulted in decreased luciferase expression in control cells, as previously shown (14). Increased Bα expression strongly decreased transcription from the cyclin A promoter, in both the presence and absence of added TGF-β (Fig. 6B). This enhancement of the cyclin A response due to Bα was similarly observed in Mv1Lu cells (data not shown). The effect of Bα on TGF-β-induced growth inhibition was also confirmed in stably transfected HaCaT cells. In these cells, the DNA synthesis, measured by using [3H]thymidine incorporation, was decreased two- to threefold when compared to that of control transfected cells in both the presence and absence of TGF-β (Fig. 6B, inset).
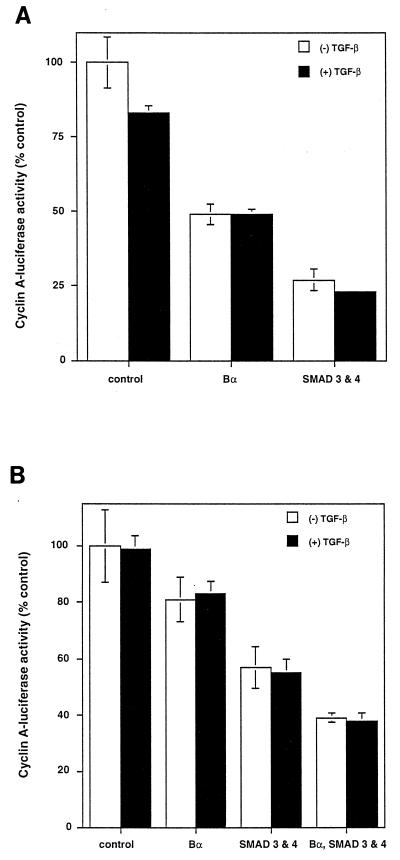
Smads have been shown to be effectors of signaling by TGF-β receptors, and Smad3 and Smad4/DPC4 together are able to induce growth inhibition (28, 49). We therefore assessed the effect of Bα overexpression on transcription from the cyclin A promoter in comparison to that of Smad3 and -4 to determine their relative effects. As shown in Fig. 7A, increased expression of Bα resulted in an antiproliferative effect that is comparable to the effect of Smad3 and -4, as assessed by using the cyclin A-luciferase assay. In these experiments, Western blotting using an anti-Myc antibody against the C-terminal tags revealed an estimated 5- to 10-fold-higher Smad expression than Bα expression (data not shown). This antiproliferative effect of Bα was also apparent in Smad4-deficient SW480.7 cells (Fig. 7B). Since Smad4 is required for the activity of Smad2 or -3, these data thus indicate that Bα can exert its antiproliferative effect independent of Smad signaling. Furthermore, the antiproliferative effects induced by Bα and by coexpressed Smad3 and -4 are additive (Fig. 7B), which is consistent with their independent ways of signaling. Finally, the antiproliferative effect of overexpressed Bα in SW480.7 cells is similar in the presence or absence of TGF-β and resembles the ligand-independent responses to overexpressed Smad3 and -4 in these cells (49) (Fig. 7B). This ligand independence stands in contrast to the TGF-β dependence of the responses to overexpressed Bα and Smad3 and -4 in HaCaT cells (Fig. 6B) (49).
FIG. 7.
Bα enhances the inhibition of cyclin A-luciferase activity independently from Smads. Bα increases the inhibition of cyclin A-luciferase activity comparably to Smad3 and -4. HaCaT (A) or Smad4-deficient SW480.7 (B) cells were cotransfected with pCal2 and pRK7 (control), pRK7-Bα, or pRK5 expression plasmids for Smad3 and -4. Luciferase expression from the cyclin A promoter was measured in the absence (−) or presence (+) of TGF-β. Bα inhibition of cyclin A-luciferase activity occurs in the absence of Smad4 and is therefore Smad independent (B), whereas Bα and Smad3-Smad4 additively inhibit cyclin A-luciferase activity in these cells.
Effect of Bα on the growth inhibition response depends on functional TGF-β receptors.
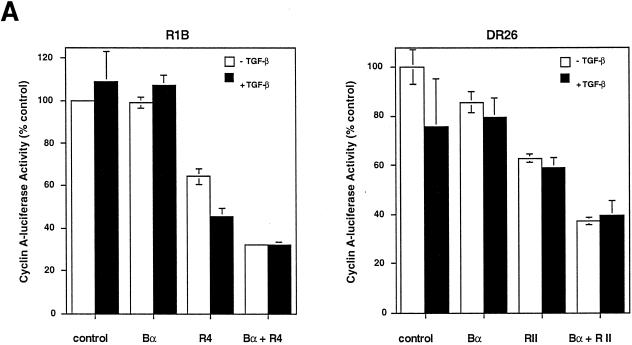
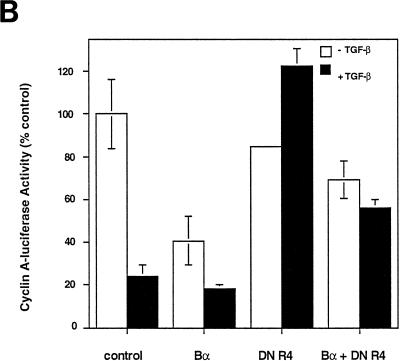
The growth inhibition effect of Bα and its enhancement of the antiproliferative effect to exogenous TGF-β in HaCaT cells (Fig. 6B) may be due to an effect of Bα, which is independent of TGF-β receptor signaling, or may depend on autocrine receptor activation by endogenous TGF-β produced by these cells (26). To distinguish between these possibilities, we analyzed the ability of Bα to inhibit the cyclin A response in mutant Mv1Lu cells, which lack functional receptors and therefore do not respond to TGF-β, i.e., R1B cells lacking type I receptors and DR26 cells lacking functional type II receptors (29). As shown in Fig. 8A, Bα did not cause growth inhibition in receptor-deficient R1B or DR26 cells, indicating that the growth inhibition response to Bα requires functional TGF-β receptors. This conclusion was confirmed by showing that reintroduction of functional type I receptors into R1B cells or type II receptors in DR26 cells restored the growth inhibition response of Bα (Fig. 8A). The sensitivity of the Bα-induced growth inhibition to functional receptors in the absence of exogenous TGF-β is consistent with the ability of cells to respond to endogenous TGF-β production and the consequent basal activation of the TGF-β receptors (37). The dependence of the growth inhibition response of Bα on TGF-β receptors was also illustrated by the effect of dominant-negative interference with TβRI function. As shown in Fig. 8B, overexpression of a cytoplasmically truncated TβRI, which inhibits receptor function (4, 14), inhibits the antiproliferative effect of Bα. Therefore, the enhancement of the growth inhibition response by Bα requires functional TGF-β receptors.
FIG. 8.
Dependence of the growth inhibition effect of Bα on functional TGF-β receptors. (A) The growth inhibition effect of Bα is TGF-β receptor dependent. R1B cells, an Mv1Lu derivative lacking functional TβRI, or DR26 cells, an Mv1Lu derivative lacking functional TβRII, were cotransfected with the pCal2 cyclin A-luciferase plasmid and pRK7 expression plasmids for Bα, TβRI/R4 (in R1B cells), or TβRII (in DR26 cells). Luciferase expression from the cyclin A promoter was measured in the absence (−) or presence (+) of TGF-β. (B) Dominant-negative inhibition of TβR1/R4 activity blocks growth inhibition by Bα. Mv1Lu cells were cotransfected with the pCal2 cyclin A-luciferase plasmid and pRK7 expression plasmids for Bα or a cytoplasmically truncated form of TβRI (DN R4) or both. Luciferase expression from the cyclin A promoter was measured in the absence or presence of TGF-β. Luciferase values, normalized for transfection efficiency, are presented relative to those of untreated, control transfected cells. Standard deviations were based on triplicate measurements.
DISCUSSION
In this study, we have shown that the WD-40 repeat-containing Bα subunit of PP2A can directly associate with the cytoplasmic domains of type I TGF-β receptors. As a result of this interaction, Bα is phosphorylated by the type I receptor kinase. This physical interaction may provide a basis for the TGF-β receptor-dependent growth inhibition effect of Bα, which complements the direct antiproliferative effect of TGF-β, and may allow the type I receptor to regulate PP2A activity in a Bα-dependent manner. These data, together with the interaction of TRIP-1 with TGF-β type II receptors, suggest that some WD-40 repeat proteins interact with serine/threonine kinase receptors.
Interaction of the Bα subunit of PP2A with TGF-β receptors.
The interaction of Bα, the WD-40 repeat subunit of PP2A, with the cytoplasmic domains of type I receptors was shown both in vitro and in vivo. Thus, purified Bα, with or without the AC core enzyme, associated directly with the cytoplasmic domains of type I receptors in vitro, and this association was confirmed in vivo. Furthermore, Bα also associated with type I receptors at the cell surface, as assessed by coimmunoprecipitation analyses of cell surface biotinylated receptors. Remarkably, Bα has a much lower affinity for the type II TGF-β receptor, even though the cytoplasmic domains of TβRII and the type I receptors have extensive sequence similarities. The association of Bα with the type I receptors parallels the association of TRIP-1, which, like Bα, has five WD-40 repeats, with TβRII (8). WD-40 repeats may be involved in the interaction with the cytoplasmic domain of the receptor, as suggested by the interaction of both Bα and Bβ with the type I receptor cytoplasmic domain, whereas B′, which lacks WD-40 repeats, does not associate. Thus, TRIP-1 interacts with the type II receptor and not with the type I receptor, whereas Bα has a much higher affinity for the cytoplasmic domains of type I receptors than for the type II receptor.
Bα bound specifically to the kinase-active and not to the kinase-inactive form of Tsk7L/R1 and TβRI/R4, again resembling the much higher affinity of TRIP-1 for kinase-active TβRII than for its kinase-inactive mutant (8). In vivo, the kinase activity and phosphorylation state of the type I receptor cytoplasmic domain are considerably enhanced by ligand-induced transphosphorylation by the type II receptor. The increased association of Bα with the type I receptor cytoplasmic domains, when the type II receptor is coexpressed, strongly suggests that Bα associates with the activated receptor complex. Auto- and transphosphorylation of the type I receptor, therefore, is likely to increase the affinity of the receptor for Bα.
The interaction of these WD-40 repeat proteins is somewhat reminiscent of the interaction of SH2 domain-containing proteins with tyrosine kinase receptors. SH2 domains are contained within several effector or adaptor proteins that mediate signaling following ligand-induced activation of these receptors. Their higher affinity for the phosphorylated tyrosines on autophosphorylated receptors allows recruitment of these signaling mediators to activated receptors and subsequent signaling events (23). TGF-β receptors, however, are serine/threonine kinase receptors with signaling mechanisms that are distinct from tyrosine kinase receptors. The interactions of TRIP-1 with the type II receptor and Bα with type I receptors, and their increased association with the autophosphorylated receptors, when compared with the kinase-inactive versions, suggest that WD-40 repeats in some proteins may mediate protein associations with activated serine/threonine kinase receptors.
The TGF-β receptors can phosphorylate Bα.
The interaction of the Bα subunit of PP2A with the type I receptor raises the possibility of functional interactions between receptor activation and the activity of PP2A. One aspect of this regulation is the phosphorylation of Bα by the type I receptor. Although phosphorylation of Bα was readily demonstrated in vitro, regulated phosphorylation of full-size Bα was not observed in vivo, possibly due to rapid dephosphorylation by PP2A. However, the use of a Bα mutant, which cannot associate with the AC core phosphatase, allowed us to visualize in vivo phosphorylation of Bα by type I receptors. Although the C subunit has previously been shown to be phosphorylated on tyrosine (6, 19), this is the first demonstration of phosphorylation of Bα.
The ability of TGF-β receptors to regulate Bα function is suggested by the observation that the inhibitory effect of Bα on cyclin A expression depends on functional receptors. This inhibition is not apparent in cells that lack functional type II or type I receptors and can be reestablished by introducing functional type I or type II receptors. Furthermore, dominant-negative interference with endogenous receptor function inhibits the antiproliferative effect of Bα.
The ability of Bα to interact with type I receptors and its association with the AC core enzyme of PP2A also raise the possibility that TGF-β receptor activation may regulate PP2A activity. Although we did not observe a regulation of the phosphatase activity of PP2A by TGF-β in untransfected HaCaT cells, increased Bα expression in transfected cells decreased the PP2A activity, and this inhibition was reversed by TGF-β (data not shown). This could be explained by increased association of Bα with the activated type I receptor and, therefore, decreased interaction with the AC core enzyme and is consistent with the inhibitory effect of Bα on PP2A activity in vitro (25). The physiological relevance of these observations is as yet unclear, especially since the use of phosphorylase A in PP2A assays is not physiologically relevant. Taken together, our data raise the possibility that Bα may couple TGF-β receptor activation with the as yet poorly understood, but complex, function of PP2A.
The Bα subunit of PP2A regulates the TGF-β response.
Our results suggest that TGF-β responsiveness can be regulated by Bα. Increased expression of Bα conferred a growth inhibition effect, which enhanced the antiproliferative response to TGF-β. No effect of Bα on expression of TGF-β or the TGF-β receptors was observed (data not shown), whereas the effect on autocrine TGF-β activation cannot be assessed. While the growth inhibition effect of Bα in the absence of added TGF-β may have suggested a response independent of TGF-β signaling, we found instead that this activity requires signaling by the receptors. This conclusion is based on our results with cells that lack functional receptors and with cells in which we overexpressed a dominant-negative mutant of the type I receptor (Fig. 8). Thus, the decreased cell proliferation induced by Bα in the absence of added TGF-β is due to a sensitivity of the cells to endogenously produced TGF-β and the consequent basal activation of the TGF-β receptors (37). Finally, the dependence of the growth inhibition effect of Bα on functional TGF-β receptors also supports the notion that TGF-β receptors have the ability to regulate the activity of Bα.
Although the regulation of the TGF-β response by Bα may conceptually be due to a Bα-mediated recruitment of the phosphatase to the activated receptor complex, which could then alter the phosphorylation of the receptors, we did not detect a change in phosphorylation of TβRI/R4 or TβRII with increased Bα expression. Changes in the pattern of phosphorylated amino acids, however, cannot be excluded. A possible dissociation of receptor-associated Bα from the PP2A core enzyme or the fact that the receptors may not serve as PP2A substrates may explain the unchanged phosphorylation level of the receptors.
The effect of Bα on the growth inhibition response of TGF-β complements the role of Smads as effectors of TGF-β receptor signaling. Smads function as transcriptional activators that induce the expression of various genes (11, 33). Since the transcription of several genes is induced by Smads (28, 49), Smads may induce growth inhibition by inducing transcription of the cdk inhibitors p15 and p21 (20, 41) in response to TGF-β. Overexpression of Bα induces growth inhibition to a level comparable to that of overexpression of Smads and, like the Smads (49), the effect of Bα on growth inhibition depends on receptor activity. Furthermore, the antiproliferative effect of Bα does not depend on Smad4, suggesting that TGF-β receptor activation may induce two parallel pathways that lead to the antiproliferative response, one propagated by Smad proteins and the other one propagated through Bα. Although the mechanism of the receptor-dependent growth inhibition by Bα is not known, one possibility is that it acts through the ability of PP2A to regulate MAP kinase activity, especially since PP2A is a major enzyme involved in dephosphorylating MAP kinase (1). Therefore, altered PP2A activity following TGF-β receptor activation might contribute to growth inhibition by deactivating this growth stimulatory pathway and, thus, complement the direct induction of growth inhibition by Smads. Moreover, a possible regulation of PP2A activity by TGF-β may also directly affect the cell cycle, which would be consistent with the observed role of PP2A in cell cycle control (9, 36).
In summary, we have demonstrated a physical interaction between the WD-40 repeat Bα subunit of PP2A and TGF-β type I receptors. This interaction results in phosphorylation and regulation of Bα. Conversely, Bα cooperates with the growth inhibition signaling by TGF-β in a receptor-dependent manner.
ACKNOWLEDGMENTS
The research was sponsored by NIH grants CA63101 to R.D. and GM49505 to M.C.M., a postdoctoral training grant from the Cardiovascular Research Institute at UCSF and a postdoctoral fellowship from the American Heart Association to I.G.-P, and a postdoctoral fellowship from the American Heart Association (Texas Affiliate) to C.K.
We thank Joan Massagué for the Mv1Lu mutant cells and the 3TP-luciferase plasmid and Norbert Fusenig for HaCaT cells. We also thank Lisa Choy, Tony DeFranco, Ellen Filvaroff, and Xin-Hua Feng for critical reading of the manuscript.
REFERENCES
- 1.Alessi D R, Gomez N, Moorhead G, Lewis T, Keyse S M, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol. 1995;5:283–295. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- 2.Andersson S, Davis D L, Dahlback H, Jornvall H, Russell D W. Cloning, structure, and expression of the mitrochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- 3.Bassing C H, Yingling J M, Howe D J, Wang T, He W W, Gustafson M L, Shah P, Donahoe P K, Wang X-F. A transforming growth factor β type I receptor that signals to activate gene expression. Science. 1993;263:87–89. doi: 10.1126/science.8272871. [DOI] [PubMed] [Google Scholar]
- 4.Brand T, Schneidert M D. Inactive type II and type I receptors for TGF β are dominant inhibitors of TGF beta-dependent transcription. J Biol Chem. 1995;270:8274–8284. doi: 10.1074/jbc.270.14.8274. [DOI] [PubMed] [Google Scholar]
- 5.Cairns J, Qin S, Philp R, Tan Y H, Guy G R. Dephosphorylation of the small heat shock protein Hsp27 in vivo by protein phosphatase 2A. J Biol Chem. 1994;269:9176–9183. [PubMed] [Google Scholar]
- 6.Chen J, Parsons S, Brautigan D L. Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J Biol Chem. 1994;269:7957–7962. [PubMed] [Google Scholar]
- 7.Chen R-H, Derynck R. Homomeric interactions between the type II TGF-β receptors. J Biol Chem. 1994;269:22868–22874. [PubMed] [Google Scholar]
- 8.Chen R-H, Miettinen P J, Maruoka E M, Choy L, Derynck R. A WD-domain protein that is associated with and phosphorylated by the type II TGF-β receptor. Nature. 1995;377:548–552. doi: 10.1038/377548a0. [DOI] [PubMed] [Google Scholar]
- 9.Clarke P R, Hoffmann I, Draetta G, Karsenti E. Dephosphorylation of cdc25-C by a type-2A protein phosphatase: specific regulation during the cell cycle in Xenopus egg extracts. Mol Biol Cell. 1993;4:397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol. 1991;201:389–397. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- 11.Derynck R, Zhang Y. Intracellular signalling: the mad way to do it. Curr Biol. 1996;6:1226–1229. doi: 10.1016/s0960-9822(96)00702-6. [DOI] [PubMed] [Google Scholar]
- 12.Derynck R, Feng X-H. TGF-β receptor signaling. BBA Rev Cancer, 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 13.Ebner R, Chen R-H, Shum L, Lawler S, Zioncheck T, Lopez A R, Derynck R. Cloning of a type I TGF-β receptor and its effect on TGF-β binding to the type II receptor. Science. 1993;260:1344–1348. doi: 10.1126/science.8388127. [DOI] [PubMed] [Google Scholar]
- 14.Feng X-H, Filvaroff E H, Derynck R. Transforming growth factor-β (TGF-β)-induced down-regulation of cyclin A expression requires a functional TGF-β receptor complex. J Biol Chem. 1995;270:24237–24245. doi: 10.1074/jbc.270.41.24237. [DOI] [PubMed] [Google Scholar]
- 15.Franzén P, ten Dijke P, Ichijo H, Yamashita H, Schulz P, Heldin C-H, Miyazono K. Cloning of TGF-β type I receptor that forms a heteromeric complex with the TGF-β type I receptor. Cell. 1993;75:681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- 16.Geng Y, Weinberg R A. Transforming growth factor β effects on expression of G1 cyclins and cyclin-dependent protein kinases. Proc Natl Acad Sci USA. 1993;90:10315–10319. doi: 10.1073/pnas.90.21.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyette M C, Cho K, Fasching C L, Levy D B, Kinzler K W, Paraskeva C, Vogelstein B, Stanbridge E. Progression of colorectal cancer is associated with multiple tumor suppressor gene defects but inhibition of tumorigenicity is accomplished by correction of any single defect via chromosome transfer. Mol Cell Biol. 1992;12:1387–1395. doi: 10.1128/mcb.12.3.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graycar J L, Miller D A, Arrick B A, Lyons R M, Moses H L, Derynck R. Human transforming growth factor-β3: recombinant expression, purification, and biological activities in comparison with transforming growth factors-β1 and -β2. Mol Endocrinol. 1989;3:1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
- 19.Guy G R, Philp R, Tan Y H. Activation of protein kinases and inactivation of protein phosphatase 2A in tumor necrosis factor and interleukin-1 signal-transduction pathways. Eur J Biochem. 1995;229:503–511. [PubMed] [Google Scholar]
- 20.Hannon G J, Beach D. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 1994;371:257–260. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 21.Healy A M, Zolnierowicz S, Stapleton A E, Goebl M, DePaoli-Roach A A, Pringle J R. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol Cell Biol. 1991;11:5767–5780. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedman K, Kurkinen M, Alitalo K, Vaheri A, Johansson S, Hook M. Isolation of the pericellular matrix of human fibroblast cultures. J Cell Biol. 1979;81:83–91. doi: 10.1083/jcb.81.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heldin C-H. Protein tyrosine kinase receptors. Cancer Surv. 1996;27:7–24. [PubMed] [Google Scholar]
- 24.Heldin C-H, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 25.Kamibayashi C, Estes R, Lickteig R L, Yang S-I, Craft C, Mumby M C. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J Biol Chem. 1994;269:20139–20148. [PubMed] [Google Scholar]
- 26.Kato M, Ishizaki A, Hellman U, Wernstedt C, Kyogoku M, Miyazono K, Heldin C-H, Funa K. A human keratinocyte cell line produces two autocrine growth inhibitors, transforming growth factor-β and insulin-like growth factor binding protein-6, in a calcium- and cell density-dependent manner. J Biol Chem. 1995;270:12373–12379. doi: 10.1074/jbc.270.21.12373. [DOI] [PubMed] [Google Scholar]
- 27.Keeton M R, Curriden S A, van Zonneveld A-J, Loskutoff D J. Identification of regulatory sequences in the type I plasminogen activator inhibitor gene responsive to transforming growth factor β. J Biol Chem. 1991;266:23048–24052. [PubMed] [Google Scholar]
- 28.Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Partnership between DPC4 and SMAD proteins in TGF-β signalling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 29.Laiho M, Weis F M B, Massague J. Concomitant loss of transforming growth factor (TGF)-β receptor types I and II in TGF-β-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem. 1990;265:18518–18524. [PubMed] [Google Scholar]
- 30.Laiho M, Weis F M B, Boyd F T, Ignotz R A, Massagué J. Responsiveness to transforming growth factor-β (TGF-β) restored by genetic complementation between cells defective in TGF-β receptors I and II. J Biol Chem. 1991;266:9108–9112. [PubMed] [Google Scholar]
- 31.Lin H Y, Wang X-F, Ng-Eaton E, Weinberg R A, Lodish H F. Expression cloning of the TGF-β type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992;68:775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Ventura F, Doody J, Massagué J. Human type II receptor for bone morphogenetic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massagué J, Hata A, Liu F. TGF-β signalling through the Smad pathway. Trends Cell Biol. 1997;7:187–192. doi: 10.1016/S0962-8924(97)01036-2. [DOI] [PubMed] [Google Scholar]
- 34.Mayer R E, Hendrix P, Cron P, Matthies R, Stone S R, Goris J, Merlevede W, Hofsteenge J, Hemmings B A. Structure of the 55kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry. 1991;30:3589–3597. doi: 10.1021/bi00229a001. [DOI] [PubMed] [Google Scholar]
- 35.Mayer-Jaekel R E, Ohkura H, Gomes R, Sunkel C E, Baumgartner S, Hemmings B A, Glover D M. The 55 kd regulatory subunit of drosophila protein phosphatase 2A is required for anaphase. Cell. 1993;72:621–633. doi: 10.1016/0092-8674(93)90080-a. [DOI] [PubMed] [Google Scholar]
- 36.Mayer-Jaekel R E, Ohkura H, Ferrigno P, Andjelkovic N, Shiomi K, Uemura T, Glover D M, Hemmings B M. Drosophila mutants in the 55 kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J Cell Sci. 1994;107:2609–2616. doi: 10.1242/jcs.107.9.2609. [DOI] [PubMed] [Google Scholar]
- 37.McCaffrey T A, Falcone D J, Brayton C F, Agarwal L A, Welt F G, Weksler B B. Transforming growth factor-β activity is potentiated by heparin via dissociation of the transforming growth factor-β/α2-macroglobulin inactive complex. J Cell Biol. 1989;109:441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCright B, Virshup D M. Identification of a new family of protein phosphatase 2A regulatory subunits. J Biol Chem. 1995;270:26123–26128. doi: 10.1074/jbc.270.44.26123. [DOI] [PubMed] [Google Scholar]
- 39.Miettinen P J, Ebner R, Lopez A R, Derynck R. TGF-β-induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neer E J, Schmidt C J, Nambudripad R, Smith T F. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- 41.Reynisdottir I, Polyak K, Iavarone A, Massagué J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 42.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 43.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein kinase 2A stimulates the MAP kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 44.Sontag E, Nunbhakdi-Craig V, Bloom G S, Mumby M C. Novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol. 1995;128:1131–1144. doi: 10.1083/jcb.128.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanabe O, Nagase T, Murakami T, Nozaki H, Usui H, Nishito Y, Hayashi H, Kagamiyama H, Takeda M. Molecular cloning of a 74-kDa regulatory subunit (B") of human protein phosphatase 2A. FEBS Lett. 1996;379:107–111. doi: 10.1016/0014-5793(95)01500-0. [DOI] [PubMed] [Google Scholar]
- 46.Tehrani M A, Mumby M C, Kamibayashi C. Identification of a novel protein phosphatase 2A regulatory subunit highly expressed in muscle. J Biol Chem. 1996;271:5164–5170. doi: 10.1074/jbc.271.9.5164. [DOI] [PubMed] [Google Scholar]
- 46a.Tehrani, M., and M. Mumby. Unpublished data.
- 47.Wrana J L, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, Wang X-F, Massagué J. TGF-β signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 48.Wrana J L, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Feng X-H, Wu R-Y, Derynck R. Receptor-associated Mad homologues synergize to induce TGF-β response. Nature. 1996;382:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]