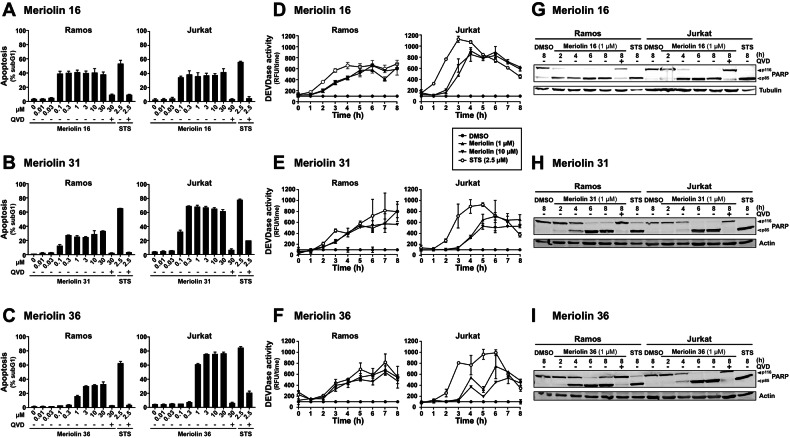
Fig. 3. Meriolin 16, 31, and 36 induce apoptosis in rapid kinetics in leukemia and lymphoma cells.
A–C About 5 × 105 Ramos lymphoma or Jurkat leukemia cells were treated with increasing concentrations of meriolin 16 (A), 31 (B), and 36 (C) or 2.5 µM staurosporine (STS), either alone or in combination (pre- and cotreatment) with the pan-caspase inhibitor QVD (10 µM). After 24 h of incubation, apoptosis-related DNA degradation was detected via flow-cytometric measurement of propidium iodide-stained apoptotic hypodiploid nuclei [25]. Error bars = SD of triplicates. D–F About 5 × 105 Ramos or Jurkat cells were treated with 1 or 10 µM of meriolin 16 (D), 31 (E), and 36 (F) or 2.5 µM staurosporine (STS) for up to 8 h. Subsequently, caspase-3 activity was determined by measurement of the fluorescence of the profluorescent caspase-3 substrate DEVD-AMC in a spectrofluorometer. Error bars = SD of triplicates. G–I About 1 × 106 Ramos or Jurkat cells were treated with 1 µM of meriolin 16 (G), 31 (H), and 36 (I), 0.1% (v/v) DMSO (solvent control), or 2.5 µM staurosporine (STS) for the indicated time periods, either alone or in combination (pre- and cotreatment) with the pan-caspase inhibitor QVD (10 µM). Cleavage of the caspase substrate PARP was detected by immunoblotting. Solid arrowheads indicate the uncleaved form of PARP (p116); open arrowheads indicate the cleaved form (p85). Immunoblotting for tubulin or β-actin was used as a loading control.