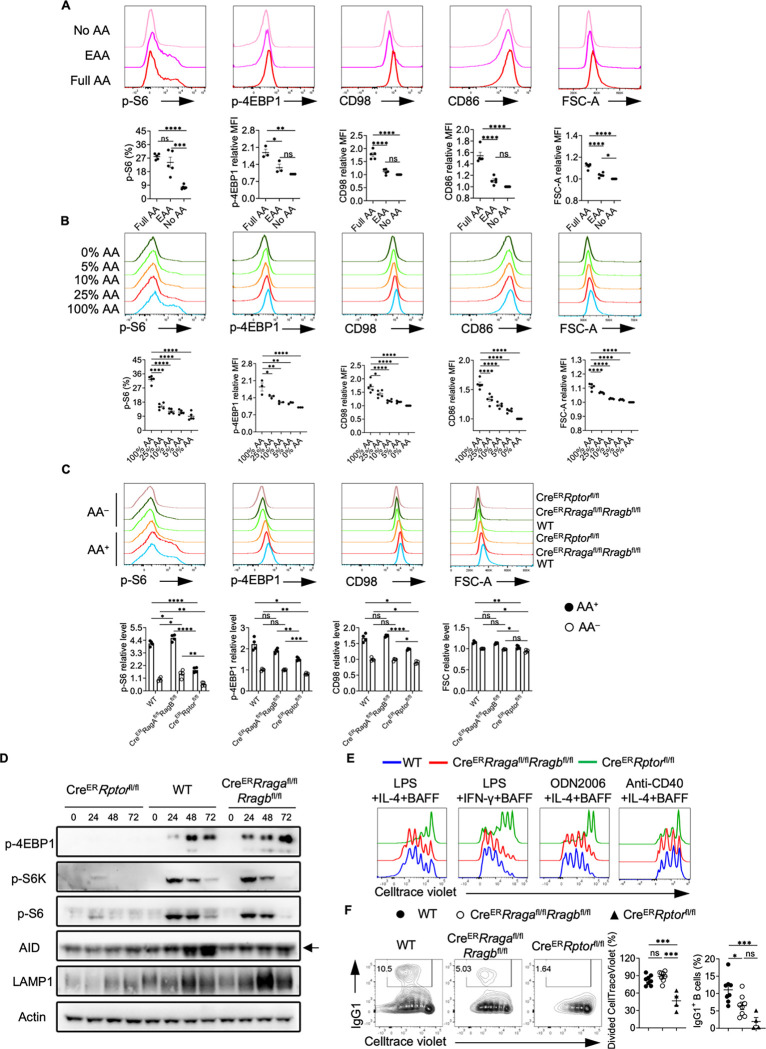
Figure 1. Amino acids modulate mTORC1 independent of Rag-GTPases.
(A) B cells were purified and cultured in no amino acids (No AA; n = 5) medium, essential amino acids (EAA; n = 5) medium, or full amino acids (Full AA; n = 5) medium with LPS/IL-4/BAFF overnight. CD98, CD86, p-4EBP1, p-S6 and FSC-A levels were measured by flow cytometry. (B) B cells were cultured in the medium with the indicated concentrations of amino acids with LPS/IL-4/BAFF overnight. CD98, CD86, p-4EBP1, p-S6 and FSC-A levels were measured by flow cytometry. N = 3–5. (C-F) Tamoxifen was administered to animals intraperitoneally daily for 4 consecutive days. Splenic B cells were purified 7 days after the last tamoxifen injection. (C) B cells were stimulated with LPS/IL-4/BAFF overnight in full AA or no AA medium. p-4EBP1, p-S6, CD98 and FSC-A levels were measured by flow cytometry. CreER control (WT) mice (n = 4), CreERRragafl/flRragbfl/fl mice (n = 4), CreERRptorfl/fl mice (n = 4). (D) B cells were stimulated with LPS/IL-4/BAFF in full AA medium for the indicated time, and cell lysates were prepared for immunoblotting to detect p-4EBP1, p-S6, p-S6K, AID and LAMP1. β-actin was used as the loading control. Arrow indicates non-specific bands. (E) B cells were labeled with CellTrace violet (CTV) and stimulated with LPS/IL-4/BAFF, LPS/IFN-γ/BAFF, ODN2006/IL-4/BAFF, or Anti-CD40/IL-4/BAFF in complete RPMI1640 medium for 72 h. CTV dilution was measured by flow cytometry. (F) B cells were stimulated with LPS/IL-4/BAFF for 72 h, and IgG1 expression was examined by flow cytometry. Right, summary of the percentages of divided cells and IgG1+ B cells. WT mice (n = 8), CreERRragafl/flRragbfl/fl mice (n = 8), CreERRptorfl/fl mice (n = 4). Error bars represent mean ± SEM. ns, not significant. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, one-way ANOVA (A, B and F), or two-way ANOVA (C).