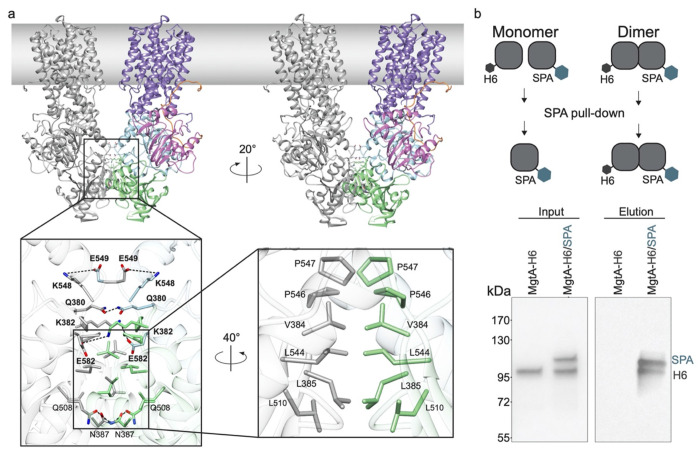
Fig 2. The dimer interface is formed by both hydrophobic and polar interactions.
a, Side view of the overall dimer structure with the left monomer in gray and the right monomer colored as in Fig. 1. A close-up view of the extensive dimer interface between the two N and P subdomains with sidechain residues displaying charge interactions across the dimer interface. Rotation of the structure highlights hydrophobic interactions at the dimer interface. Molecular dynamic simulations (see Extended Data Movies 5 and 6) show consistent interactions across the dimer interface between K382-E582 (64%), Q380-Q380 (87%), and K548-E549 (88%) shown in bold. b, Co-purification of two differentially tagged MgtA derivatives, one tagged with His6 and the other tagged with the larger SPA tag as shown in the top schematic reveals copurification of the two proteins (right elution panel). Proteins were visualized by Western blot analysis using MgtA antibodies.