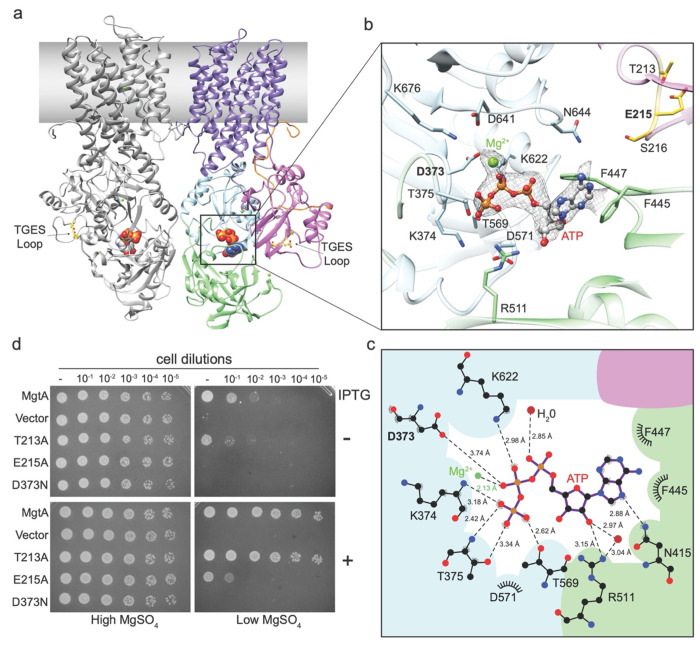
Fig 3. The nucleotide binding pocket of MgtA is accessible in the dimeric state.
a, Side view of the MgtA dimer with the left monomer in gray and the right monomer colored as in Fig. 1, highlighting the ATP molecule represented in spheres located in between the soluble A domain and P and N subdomains and the dephosphorylation TGES loop in yellow. b, A close-up view of the ATP binding site highlighting residues in close proximity to the Mg-ATP molecule which is shown in a ball and stick representation and the cryo-EM map density in gray mesh. Residues from the TGES loop involved in dephosphorylation and located in the A domain are colored yellow. c, Distances from ATP to amino acids, water molecules and Mg2+ ion in the nucleotide binding pocket of MgtA from E. coli, determined using LIGPLOT+ 88. d, MgtAD373N is unable to completement a Mg2+-auxotrophic E. coli strain indicating this mutant transporter does not translocate Mg2+ ions. D373 is the residue that is being phosphorylated upon ATP hydrolysis. MgtAE215A is only partially able to complement upon overexpression. E215 is part of the TGES loop involved in dephosphorylation. Overnight cultures were serial diluted and spotted onto LB agar plates supplemented with high (100 mM) or low (1 mM) MgSO4 with (+) and without (−) 0.1 mM IPTG for induction and grown at 37°C (also see Extended Data Fig. 21).