Abstract
BACKGROUND:
Agitation is a common presentation within emergent departments (EDs). Agitation during pregnancy should be treated as an obstetric emergency, as the distress may jeopardize both the patient and fetus. The safety of psychotropic medications in the reproductive age female has not been well established. This review aimed to explore a summary of general agitation recommendations with an emphasis on ED management of agitation during pregnancy.
METHODS:
A literature review was conducted to explore the pathophysiology of acute agitation and devise a preferred treatment plan for ED management of acute agitation in the reproductive age or pregnant female.
RESULTS:
While nonpharmacological management is preferred, ED visits for agitation often require medical management. Medication should be selected based on the etiology of agitation and the clinical setting to avoid major adverse effects. Adverse effects are common in pregnant females. For mild to moderate agitation in pregnancy, diphenhydramine is an effective sedating agent with minimal adverse effects. In moderate to severe agitation, high-potency typical psychotropics are preferred due to their neutral effects on hemodynamics. Haloperidol has become the most frequently utilized psychotropic for agitation during pregnancy. Second generation psychotropics are often utilized as second-line therapy, including risperidone. Benzodiazepines and ketamine have demonstrated adverse fetal outcomes.
CONCLUSION:
While randomized control studies cannot be ethically conducted on pregnant patients requiring sedation, animal models and epidemiologic studies have demonstrated the effects of psychotropic medication exposure in utero. As the fetal risk associated with multiple doses of psychotropic medications remains unknown, weighing the risks and benefits of each agent, while utilizing the lowest effective dose remains critical in the treatment of acute agitation within the EDs.
Keywords: Agitation, Pregnancy, Haloperidol, Ketamine, Benzodiazepines
INTRODUCTION
Agitation, as defined by the American Association for Emergency Psychiatry BETA project, is “an extreme form of arousal that is associated with increased verbal and motor activity.”[1] The pathophysiology of agitation remains a complex topic, as it presents in an episodic pattern and varies among individuals with an underlying primary psychiatric illness or substance use. Excessive bottom-up activation occurs in response to environmental stimuli or threat overestimation, with inappropriate behavioral control from higher cortical centers. Threat overestimation results in activation of the hypothalamic-pituitary adrenal axis and subsequent release of excitatory neurotransmitters (glutamate, acetylcholine, norepinephrine, and dopamine).[2] High doses of norepinephrine in the prefrontal cortex impair working memory and executive functioning.[3] Dopamine increases amygdala excitation and exacerbates fear responses, paranoia and delusions. Agitated states also result in decreased levels of serotonin and gamma-aminobutyric acid (GABA), the two major inhibitory neurotransmitters of the central nervous system. The higher cortical centers controlled by serotonin and GABA are thus unable to modulate dysphoric reactions.[2]
Agitation is an increasingly common presentation within emergency departments (EDs), psychiatry units, and long-term care facilities. The prevalence of agitation within EDs compromises around 2.6% of total patient encounters.[4] Traditionally, all agitated patients were immediately restrained, secluded, or given high-dose psychotropic medications. Currently, verbal de-escalation is typically trialed upon initial presentation. Noise and other distracting factors should be limited, while the patient is provided comfort and empathy. When agitation is severe and persists despite these interventions, medications must be administered for safety concerns. It is reported that 3–20 per 1,000 ED visits for agitation are medically managed, with over 50% given involuntarily.[5] The medications administered voluntarily or involuntarily should differ based on the etiology of the agitation and the clinical setting. Physiologic stress as a response to extrinsic factors such as the death of a family member, loss of an occupation, financial insecurity, or other life stressors, has been shown to be a leading cause of acute agitation.[2] Other causes include alcohol use or withdrawal, drug intoxication or withdrawal, underlying primary psychiatric or mental health disorders, and underlying medical causes. Common primary mental health disorders that fall into this category include bipolar disorder, schizophrenia, autism spectrum disorder, intellectual disability, anxiety, and depression. Underlying medical etiologies include head trauma, sepsis, dementia, delirium, electrolyte abnormalities, endocrine disorders, postictal states, and toxic exposures.[2] Understanding the etiology of agitation is critical in proper patient care as adverse reactions to sedation have been reported in up to 15.9% of patients medically managed in the ED.[6] Adverse reactions include hypoxia, hypotension, bradycardia, QTc prolongation, and airway obstruction. These effects are commonly observed in the elderly population (65 years or older), patients with alcohol intoxication, and individuals prescribed multiple sedative medications.[6]
An additional population requiring careful monitoring for adverse reactions to sedation includes the pregnant female. Acute agitation during pregnancy should be treated as an obstetric emergency, as the distress may jeopardize both the patient and the fetus, resulting in preterm labor, placental abnormalities, postnatal death and spontaneous abortion. In this narrative review article, a summary of general agitation recommendations, with an emphasis on agitation during pregnancy, will be explored. Understanding the safety of psychotropic medications in the reproductive age or pregnant female is critical in optimizing patient care and devising standardized treatment protocols for ED management of acute agitation.
METHODS
A narrative review was conducted. Medical literature published in any language since 1960 until 2022 was identified utilizing MEDLINE/PubMed and the Cochrane Library. Additional references were collected through use of the reference lists of published articles identified by the selected databases. Initial search terms included typical and atypical psychotropic medication, diphenhydramine, ketamine, haloperidol, and benzodiazepines. Articles were then subcategorized into general drug information, mechanism of action, contraindications, and selective utilization in the management of acute agitation. A separate search was conducted to identify the reported teratogenicity and adverse effect profiles of the selected drug classes. There were no methodological limitations in relation to the initial acquisition and analysis of data. As literature pertaining to human and in-vitro subjects was limited, articles referencing animal models and epidemiologic studies were included. Articles were screened by abstract and then selected on the basis of full-text publications. Sample size and generalizability were taken into account in article selection. The author possessed a distinction in research and was the only reviewer who performed selection and data extraction. A total of 39 research articles were included in the final manuscript.
RESULTS
Agitation: drug overview
There are four major drug classes utilized in the management of acute agitation: typical psychotropics (first-generation), atypical psychotropics (second-generation), benzodiazepines, and ketamine. Typical psychotropics mainly include haloperidol and droperidol. Haloperidol, a high potency typical psychotropic, exerts its effects through antagonism of dopamine D2 receptors. Haloperidol can be administered orally (PO), intravenously (IV), or intramuscularly (IM), with an average time to sedation of around 25–28 min. Haloperidol has demonstrated the greatest utility in agitation secondary to delirium.[2] The primary adverse effects are extrapyramidal symptoms (EPS), including akathisia and dystonia. These effects can be mitigated by the addition of an anticholinergic agent, such as diphenhydramine or benztropine. Haloperidol may also result in QTc prolongation. Alternatively, droperidol, an analog of haloperidol, can be administered IV or IM with an average time to sedation around 15–30 min. Droperidol can also result in QTc prolongation and EPS. However, when compared to haloperidol, droperidol has been shown to require less frequent dosing with similar effects on mental status. [7]
Atypical psychotropics, such as clozapine, olanzapine, quetiapine, lurasidone, and ziprasidone have a higher affinity for serotonin 5-HT2 receptors and a lower affinity for D2 receptors. Consequently, they are capable of exerting therapeutic effects with a lower risk of EPS. Risperidone has equal affinity for 5-HT2A and D2 receptors, while aripiprazole is an antagonist of 5-HT2A and partial agonist of D2 receptors. Risperidone and aripiprazole have demonstrated improvement in repetitive behaviors in patients with agitation secondary to frustration or communication difficulties, as seen in autism spectrum disorder.[2] The effects on aggression, irritability and restlessness stem from antagonism of the dopamine receptors. The effects on communication skills, emotional and social interaction, and restricted activity patterns stem from antagonism of the serotonin receptors.[2]
Only ziprasidone, olanzapine, risperidone, and aripiprazole are available as parenteral agents.[8] Ziprasidone is available PO or IM with time to onset 15–20 min. Ziprasidone is associated with high risk of QTc prolongation. Systemic reviews involving ziprasidone have yet to be published.[9] Olanzapine can be given IM or IV for severe agitation, with a peak concentration at 15–45 min. Intramuscular administration of both ziprasidone and olanzapine requires reconstitution and therefore has limited efficacy in acute agitation. Olanzapine should not be combined with benzodiazepines or in patients suspected of having anticholinergic overdose due to the risk of hypotension and cardiopulmonary depression. For agitation secondary to a primary psychiatric condition, the World Federation of Societies of Biological Psychiatry (WFSBP) recommends the use of typical or atypical psychotropics to rapidly calm, without overtly sedating, the individual and address the underlying dopaminergic psychosis.[10] Typical and atypical psychotropics are further recommended by the WFSBP for agitation secondary to alcohol intoxication.[10] Haloperidol is particularly well-studied in treating agitation associated with alcohol intoxication. [2]
Compared to typical and atypical psychotropics, benzodiazepines exert a significantly enhanced sedating effect. Benzodiazepines increase the affinity of GABA to GABA-A receptors. GABA is the most abundant central nervous system (CNS) inhibitory neurotransmitter. Benzodiazepines thus cause significantly pronounced and dose-dependent CNS depression, respiratory depression, anxiolysis, sensory and motor impairments, and anterograde amnesia. The two main benzodiazepines utilized for acute agitation include midazolam and lorazepam. Midazolam can be given intranasally, IV, IM, rectally (PR), or PO, with onset varying from 1–5 min IV to 13–18 min IM. Lorazepam is longer acting with an average time to sedation around 32 min. Midazolam is typically preferred over lorazepam for acute agitation due to its quicker time of onset and shorter half-life. Nobay et al[11] demonstrates shorter time to onset of sedation and time to arousal with midazolam use; however, the efficacy among lorazepam, haloperidol, and midazolam was similar.
When agitation is secondary to alcohol withdrawal, benzodiazepine withdrawal, or stimulant toxicity, benzodiazepines are considered first-line therapy rather than psychotropics due to their CNS depressant effects.[2] Furthermore, the American College of Emergency Physicians (ACEP) recommends the use of benzodiazepines or conventional psychotropics for acute agitation requiring rapid de-escalation.[12] The combination of benzodiazepines and psychotropics is a common practice that has not consistently shown improvement in the control of agitation.[6,13] While the combination of the two agents increases the risk of sedation, it may also allow the mitigation of each drug’s individual adverse effects. Through significant CNS depression, benzodiazepines can help mitigate the EPS of typical psychotropics. Similarly, haloperidol may mitigate the paradoxical agitation associated with benzodiazepine use.[14] In contrast, atypical psychotropics, particularly olanzapine, should not be used in combination with benzodiazepines due to significant drug interactions potentially resulting in hypotension, bradycardia, and respiratory depression.
Ketamine is an emerging drug utilized in acute agitation. Ketamine is a nonbarbiturate dissociated anesthetic that functions as a noncompetitive N-methyl-D-aspartate (NMDA) and glutamate receptor antagonist. By antagonizing NMDA receptors, ketamine rapidly controls symptoms of depression and acute suicidal ideation. Ketamine also functions as a partial agonist on opiate receptors. Ketamine thus causes a unique dissociative effect, maintaining the patient in a state of sedation, comfortable awareness, and analgesia.[15] Ketamine can be given IV or IM with an average time to sedation of 5 min. Ketamine is thus a preferred agent for the rapid control of severe and combative agitation. While the time to onset is rapid, ketamine’s effects rapidly decline at a clearance rate of 12–17 mL/(kg·h), and redosing is frequently needed within 10 to 15 min. Due to its weak sympathomimetic activity, ketamine has been noted to worsen tachycardia and hypertension in non-agitated patients. A dysphoric emergence phenomenon has also been reported in 10%–20% of adult patients sedated with ketamine.[15] The dysphoric phenomena can be treated with low doses of benzodiazepines. Other adverse effects include hypersalivation (11%), vomiting (2%–8%), laryngospasm (1%), increased rate of intubation (2%) and transient hypoxia (0%–4%).[15] The etiology of agitation should be considered prior to ketamine use. Ketamine should be avoided in patients with unclear primary psychiatric diagnoses as administration may paradoxically worsen agitation.[16] The ACEP Clinical Policy lists psychiatric illness as an absolute contraindication for ketamine sedation. Various clinical trials involving ketamine administration in patients with schizophrenia have demonstrated an increase in schizophrenic symptoms in the short term with a return to baseline within 90 min in 100% of clinical subjects.[16]
Agitation: agent of choice in pregnancy
Psychiatric emergencies are common within the perinatal and postnatal periods.[17] Major depressive disorder occurs in one in six pregnant women, and one in four women with bipolar disorder experience mood exacerbation during this time. Furthermore, increased agitation during pregnancy is correlated with increased obstetric complications, including preterm delivery, placental abnormalities, spontaneous abortion, fetal demise, and suicidality.[17] Consequently, an agitated pregnant patient should be considered an obstetric emergency.
As with all agitated patients, the initial evaluation should consist of careful consideration regarding the etiology of agitation. A broader differential approach includes consideration of hypoxia, hypercapnia, amniotic and venous thromboembolism, coagulopathy, intracranial hemorrhage, trauma, delirium, primary psychiatric disorder, alcohol intoxication or withdrawal, and drug intoxication or withdrawal. Eclampsia must also be considered in women beyond 20 weeks of gestation.
All psychotropic medications readily cross the placenta, are present in amniotic fluid, and can enter breast milk to varying degrees. While randomized control studies cannot be ethically conducted on pregnant patients requiring acute sedation, animal models and epidemiologic studies have demonstrated the effects of psychotropic medications on transmission to the fetus. See Table 1. There are three primary areas of effects that occur due to psychotropic medication taken during pregnancy: 1) teratogenicity, 2) perinatal syndromes (neonatal toxicity) and 3) postnatal behavioral sequelae. Drug exposure during the first trimester has the most significant effect on outcome, as blastogenesis and organogenesis occur during this time. Epidemiologic studies suggest that most psychotropic drugs, including typical psychotropics, atypical psychotropics, benzodiazepines, and diphenhydramine, are relatively safe during pregnancy. The absolute risk of congenital malformations from long-term use of such drugs is 2%–3% within the first trimester.[18] As such, current recommendations favor the use of a single agent at a higher dose, with higher protein binding and fewer medication interactions.
Table 1.
Five major drug classes utilized in the treatment of acute agitation, with a focus on the mechanism of action, adverse effect profiles, general recommendations for use, and use during pregnancy
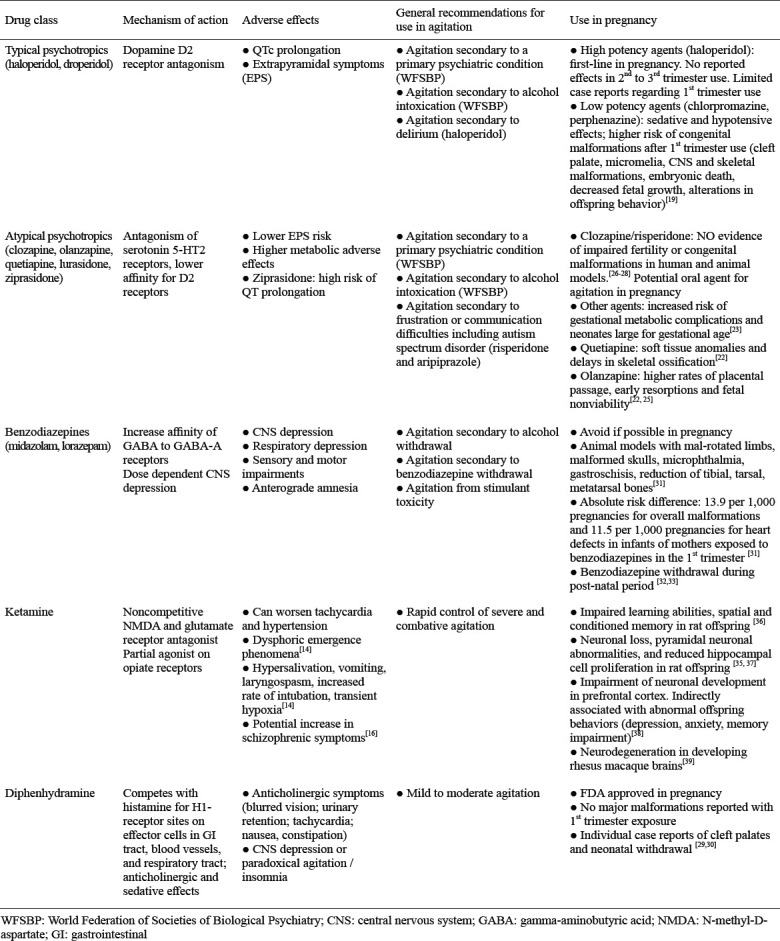
In the setting of acute agitation during pregnancy, high-potency typical psychotropics, such as haloperidol, are recommended over low-potency typical psychotropics such as chlorpromazine or perphenazine due to decreased sedative and hypotensive effects.[19] A recent meta-analysis demonstrated a higher risk of congenital malformations after first trimester exposure to low potency psychotropics which has not been demonstrated with higher potency agents, such as haloperidol.[19] Teratogenic effects include cleft palate, micromelia, and CNS and skeletal malformations. Other adverse effects include embryonic death, fetal death, decreased fetal growth, and long-lasting alterations in offspring behavior. While no congenital defects have been reported with the use of haloperidol in the second and third trimesters, conflicting data exist regarding the effects of first trimester haloperidol use. Two case reports have shown an increased risk of limb reduction defects with haloperidol use in the first trimester.[20] In contrast, Van Waes et al[21] found no association between fetal anomalies and haloperidol use in hyperemesis gravidarum during the first trimester. While no congenital abnormalities have been reported in second or third trimester Haloperidol use, an increased risk of neonatal withdrawal, EPS, and sedation have been reported in neonates of mothers exposed to Haloperidol in the second and third trimesters. EPS in neonates includes hypertonicity, tremor, restlessness, dystonia, and parkinsonism.[22] Such adverse effects were observed only in neonates of mothers exposed to repeated haloperidol use, rather than a single use. Consequently, the Clinical Consensus Guidelines published in 2001 recommend the use of haloperidol for acute agitation in pregnancy with a 76% consensus rate.[23] Furthermore, Ladavac et al[24] studied the pharmacological management of agitated pregnant women within the psychiatric emergency service and found that haloperidol alone was the most frequently utilized medication. Risperidone was the second most commonly utilized medication.
As with low-potency typical psychotropics, atypical psychotropics have been associated with an increased risk of neonatal complications. Atypical psychotropics show an increased risk of gestational metabolic complications and neonates large for gestational age.[23] In animal models, quetiapine demonstrates embryotoxic and fetotoxic effects including soft tissue anomalies and delays in skeletal ossification.[22] Similarly, olanzapine demonstrates fetotoxic effects in animal models such as early resorptions and fetal nonviability.[22] Newport et al [25] described higher rates of placental passage (72.1% ±42%), lower birth weight, and increased perinatal complications associated with olanzapine as compared to other psychotropic medications. In contrast, clozapine and risperidone demonstrate no evidence of impaired fertility or congenital malformations in human and animal models.[26-28] The placental passage of risperidone has been estimated at 49.2% ± 33.9% in human.[25] Risperidone is therefore a potential oral agent for the management of agitation in pregnancy. There is a paucity of evidence regarding long-term cognitive and behavioral outcomes in infants exposed to atypical psychotropics. However, atypical psychotropics are not generally utilized in the acute setting of severe agitation as patients frequently require IV or IM rapidly acting medications.
First-generation antihistamines, specifically diphenhydramine, are considered pregnancy safe according to the FDA. No major malformations have been reported with first-trimester exposure to antihistamines. However there are individual case reports of cleft palates and neonatal withdrawal that have been documented.[29,30] With the paucity of adverse effects reported, diphenhydramine is an excellent agent of choice in mild to moderate agitation.
As discussed above, benzodiazepines are often administered for rapid de-escalation of acute agitation. Lorazepam and midazolam are two of the most utilized benzodiazepines, yet both should be avoided in pregnant patients. Benzodiazepines readily cross the placenta, as evidenced by high levels of the drugs in umbilical cord blood. Animal models treated with benzodiazepines demonstrate occasional anomalies including malrotated limbs, malformed skulls, microphthalmia, gastroschisis, and reduction of tibial, tarsal, and metatarsal bones.[31] In a population-based cohort study by Noh et al., the absolute risk difference for overall malformations was 13.9 per 1,000 pregnancies and 11.5 per 1,000 pregnancies for heart defects in infants of mothers exposed to benzodiazepines in the first trimester. [31] The incidence of malformations and heart defects was higher among women over 35 years old, those with multi-fetal pregnancy, and those with a history of epilepsy. The risk of overall malformations was comparable between short- and long-acting benzodiazepines.[31]
In addition, infants of mothers with several weeks of benzodiazepine ingestion have shown withdrawal symptoms during the postnatal period. Symptoms include hypoactivity, hypotonia, hypothermia, respiratory depression, apnea, and feeding difficulties (“floppy-baby syndrome”). Such symptoms are more pronounced in neonates of mothers exposed to benzodiazepines in the third trimester or at delivery. [32, 33] Current literature has not investigated the risk associated with a one-time acute exposure in the EDs.
While ketamine is an emerging agent for the control of severe agitation, evidence has shown that ketamine easily traverses the blood-placental barrier from mother to fetus.[34] Dong et al[35] demonstrated that ketamine exposure in utero results in reduced neuronal development in offspring. Li et al[36] demonstrated impaired learning abilities, spatial and conditioned memory in offspring of female rats anesthetized with 3 h of IV ketamine injection. Similarly, Zhao et al[37] demonstrated neuronal loss, pyramidal neuronal abnormalities, and reduced hippocampal cell proliferation in the offspring of pregnant rats exposed to ketamine in the second trimester. In a later study by Zhao et al,[38] maternal ketamine exposure resulted in cell apoptosis and neuronal loss specifically in the prefrontal cortex of the fetal brain. By impairing neuronal development of the prefrontal cortex, ketamine is indirectly associated with abnormal behaviors in offspring, including depression, anxiety, and memory impairment. In addition to rat models, the neurotoxic effects of maternal ketamine exposure have been demonstrated in non-human primates. Brambrink et al[39] demonstrated that ketamine induced neurodegeneration in developing fetal and neonatal rhesus macaque brains. A ketamine exposure duration of 5 h was sufficient to induce significant neuronal apoptosis. Neuronal loss was 2.2 times greater in fetal brains than in neonates. Given the neurotoxic and neurobehavioral effects demonstrated in animal models and the lack of adequate human data, the use of ketamine is not recommended for use during pregnancy.
In the case that medical management is not sufficient to manage acute agitation, physical restraint is utilized for safety precautions. However, physical restraint can be very dangerous in pregnancy, especially in the third trimester, due to potential inferior vena caval (IVC) compression. If restraint is needed, the patient should be placed in the left lateral decubitus position or have their right side supported.[1]
Bias and limitation assessment
A comprehensive narrative review was performed on the literature regarding agitation and adverse fetal outcomes associated with ED management of acute agitation. Significant effort was made to limit selection bias with specified search criteria and inclusion of all relevant data supporting or differing from the stated theory. Despite such efforts, the study is limited in that it is not a systematic review and, thus, did not follow specified academic guidelines. Subjective methodology and author selection of data from published references may serve to limit the intrinsic scientific validity. An additional limitation includes the small sample size secondary to the lack of published data on human and in-vitro subjects. Further studies are warranted to assess long-term fetal outcomes of psychotropic medication use.
CONCLUSIONS
The safety of psychotropic medications in pregnancy has not been well established on human and thus, the risks and benefits of their use should be carefully considered. Alternative nonpharmacological strategies are preferred for mild to moderate agitation. In moderate agitation nonresponsive to verbal de-escalation, diphenhydramine is an effective sedating agent with minimal short- and long-term adverse effects. Diphenhydramine will also help mitigate extrapyramidal symptoms if psychotropics are needed. In moderate to severe agitation, high-potency typical psychotropics are preferred due to their neutral effects on hemodynamics. Haloperidol is the most frequently utilized medication, with risperidone as the second most common agent. As the fetal risk associated with multiple doses of psychotropic medications remains unknown, weighing the risks and benefits of each agent, while utilizing the lowest effective dose of a single agent remains critical. Further research is necessary to analyze the teratogenicity and long-term adverse effects associated with psychotropic medications. Identification of such risks will aid in improving patient care and devising standardized treatment protocols for ED management of acute agitation in the reproductive age and pregnant female.
Footnotes
Funding: The authors did not receive support from any organization for the submitted work.
Ethical approval: Not needed.
Conflicts of interest: All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Author contribution: We certify that all listed authors meet the journal’s specific requirements regarding the duties and responsibilities of authorship.
REFERENCES
- 1.Holloman GH, Jr, Zeller SL. Overview of project BETA:best practices in evaluation and treatment of agitation. West J Emerg Med. 2012;13(1):1–2. doi: 10.5811/westjem.2011.9.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui W, Gupta V, Huecker MR. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2023. Agitation. [Google Scholar]
- 3.Berridge CW, Spencer RC. Differential cognitive actions of norepinephrine α2 and α1 receptor signaling in the prefrontal cortex. Brain Res. 2016;(1641(Pt B)):189–96. doi: 10.1016/j.brainres.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361–70. doi: 10.1016/j.annemergmed.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Nordstrom K, Zun LS, Wilson MP, Stiebel V, Ng AT, Bregman B, et al. Medical evaluation and triage of the agitated patient:consensus statement of the American Association for Emergency Psychiatry Project Beta Medical Evaluation Workgroup. West J Emerg Med. 2012;13(1):3–10. doi: 10.5811/westjem.2011.9.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yap CYL, Taylor DM, Kong DCM, Knott JC, Taylor SE. Sedation for Acute Agitation in Emergency Department Patients:Targeting Adverse Events (SIESTA) Collaborative Study Group. Risk factors for sedation-related events during acute agitation management in the emergency department. Acad Emerg Med. 2019;26(10):1135–43. doi: 10.1111/acem.13826. [DOI] [PubMed] [Google Scholar]
- 7.Khokhar MA, Rathbone J. Droperidol for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2016;12(12):CD002830. doi: 10.1002/14651858.CD002830.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orsolini L, Tomasetti C, Valchera A, Vecchiotti R, Matarazzo I, Vellante F, et al. An update of safety of clinically used atypical antipsychotics. Expert Opin Drug Saf. 2016;15(10):1329–47. doi: 10.1080/14740338.2016.1201475. [DOI] [PubMed] [Google Scholar]
- 9.Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269–75. doi: 10.1097/PEC.0000000000000112. quiz276-8. [DOI] [PubMed] [Google Scholar]
- 10.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthøj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia - a short version for primary care. Int J Psychiatry Clin Pract. 2017;21(2):82–90. doi: 10.1080/13651501.2017.1291839. [DOI] [PubMed] [Google Scholar]
- 11.Nobay F, Simon BC, Levitt MA, Dresden GM. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11(7):744–9. doi: 10.1197/j.aem.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 12.American College of Emergency Physicians Hyperactive Delirium Task Force. ACEP Task Force Report on Hyperactive Delirium with Severe Agitation in Emergency Settings. Available at: https://www.acep.org/siteassets/new-pdfs/education/acep-task-force-report-on-hyperactive-delirium-final.pdf .
- 13.Gillies D, Sampson S, Beck A, Rathbone J. Benzodiazepines for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2013;(4):CD003079. doi: 10.1002/14651858.CD003079.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Zareifopoulos N, Panayiotakopoulos G. Treatment options for acute agitation in psychiatric patients:theoretical and empirical evidence. Cureus. 2019;11(11):e6152. doi: 10.7759/cureus.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum SB, Gupta V, Palacios JL. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2022. Ketamine. [PubMed] [Google Scholar]
- 16.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13(1):9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 17.Aftab A, Shah AA. Behavioral emergencies:special considerations in the pregnant patient. Psychiatr Clin North Am. 2017;40(3):435–48. doi: 10.1016/j.psc.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy:dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592–606. doi: 10.1176/ajp.153.5.592. [DOI] [PubMed] [Google Scholar]
- 19.Edinoff AN, Sathivadivel N, McNeil SE, Ly AI, Kweon J, Kelkar N, et al. Antipsychotic use in pregnancy:patient mental health challenges, teratogenicity, pregnancy complications, and postnatal risks. Neurol Int. 2022;14(1):62–74. doi: 10.3390/neurolint14010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal MM, Aneja A, Rahman A, Megna J, Freemont W, Shiplo M, et al. The potential risks of commonly prescribed antipsychotics:during pregnancy and lactation. Psychiatry. 2005;2(8):36–44. [PMC free article] [PubMed] [Google Scholar]
- 21.Van Waes A, Van de Velde E. Safety evaluation of Haloperidol in the treatment of hyperemesis gravidarum. J Clin Pharmacol. 1969;9(4):224–7. [Google Scholar]
- 22.Iqbal MM, Aneja A, Rahman A, Megna J, Freemont W, Shiplo M, et al. The potential risks of commonly prescribed antipsychotics:during pregnancy and lactation. Psychiatry (Edgmont) 2005;2(8):36–44. [PMC free article] [PubMed] [Google Scholar]
- 23.Allen MH, Currier GW, Hughes DH, Reyes-Harde M, Docherty JP. Expert Consensus Panel for Behavioral Emergencies. The expert consensus guideline series. Treatment of behavioral emergencies. Postgrad Med. 2001;(Spec No 1):88. quiz89-90. [PubMed] [Google Scholar]
- 24.Ladavac AS, Dubin WR, Ning A, Stuckeman PA. Emergency management of agitation in pregnancy. Gen Hosp Psychiatry. 2007;29(1):39–41. doi: 10.1016/j.genhosppsych.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Newport DJ, Calamaras MR, DeVane CL, Donovan J, Beach AJ, Winn S, et al. Atypical antipsychotic administration during late pregnancy:placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164(8):1214–20. doi: 10.1176/appi.ajp.2007.06111886. [DOI] [PubMed] [Google Scholar]
- 26.Moriarty AJ, Nance MR. Trifluoperazine and pregnancy. Can Med Assoc J. 1963;88(7):375–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Russell T. Drugs in pregnancy survey. Pract. 1963;191:775–80. [PubMed] [Google Scholar]
- 28.Ratnayake T, Libretto SE. No complications with risperidone treatment before and throughout pregnancy and during the nursing period. J Clin Psychiatry. 2002;63(1):76–7. doi: 10.4088/jcp.v63n0114c. [DOI] [PubMed] [Google Scholar]
- 29.Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Research. 2009;85(2):137–50. doi: 10.1002/bdra.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.So M, Bozzo P, Inoue M, Einarson A. Safety of antihistamines during pregnancy and lactation. Can Fam Physician. 2010;56(5):427–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Noh Y, Lee H, Choi A, Kwon JS, Choe SA, Chae J, et al. First-trimester exposure to benzodiazepines and risk of congenital malformations in offspring:a population-based cohort study in South Korea. PLoS Med. 2022;19(3):e1003945. doi: 10.1371/journal.pmed.1003945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warning:risks from concomitant use with opioids. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2016/017794s044lbl.pdf .
- 33.Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines:an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46–8. doi: 10.1016/S1701-2163(16)34772-7. [DOI] [PubMed] [Google Scholar]
- 34.Ellingson A, Haram K, Sagen N, Solheim E. Transplacental passage of ketamine after intravenous administration. Acta Anaesthesiol Scand. 1977;21(1):41–4. doi: 10.1111/j.1399-6576.1977.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 35.Dong C, Rovnaghi CR, Anand KJ. Ketamine exposure during embryogenesis inhibits cellular proliferation in rat fetal cortical neurogenic regions. Acta Anaesthesiol Scand. 2016;60(5):579–87. doi: 10.1111/aas.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Guo C, Li Y, Li L, Wang Y, Zhang Y, et al. Ketamine administered pregnant rats impair learning and memory in offspring via the CREB pathway. Oncotarget. 2017;8(20):32433–49. doi: 10.18632/oncotarget.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao T, Li Y, Wei W, Savage S, Zhou L, Ma D. Ketamine administered to pregnant rats in the second trimester causes long-lasting behavioral disorders in offspring. Neurobiol Dis. 2014;68:145–55. doi: 10.1016/j.nbd.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhao TY, Li CX, Wei W, Zhang HX, Ma DQ, Song XR, et al. Prenatal ketamine exposure causes abnormal development of prefrontal cortex in rat. Sci Rep. 2016;6:26865. doi: 10.1038/srep26865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116(2):372–84. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]


